
In chemistry, the term transition metal (or transition element) has three possible definitions:
- The IUPAC definition [1] defines a transition metal as "an element whose atom has a partially filled d sub-shell, or which can give rise to cations with an incomplete d sub-shell".
- Many scientists describe a "transition metal" as any element in the d-block of the periodic table, which includes groups 3 to 12 on the periodic table. ...
Where are non metals and metals placed on the periodic table?
The nonmetals are a group of elements located on the right side of the periodic table (except for hydrogen, which is on the top left). There are also known as non metals and non-metals.
What elements are found in transition metals?
Transition metal compounds dissolved in water exhibit a wide variety of bright colors. From left to right are shown solutions of cobalt (II) nitrate, potassium dichromate, potassium chromate, nickel (II) chloride, copper (II) sulfate, and potassium permanganate.
What groups are transition metals?
Transition metals are metallic elements in groups IB – VIIIB characterised by metallic bonding, coloured compounds, varying oxidation states and catalytic ability. Transition metals have high electric conductivity due to the free flowing outer d-orbital electrons. They have low electron affinity, low ionization energy, and low electronegativity.
Where are rare elements on a periodic table?
Rare Earth Elements in Periodic Table [Image will be Uploaded Soon] Rare earth elements, also commonly known as lanthanides are basically the elements present in the top extended row placed below the main body of the periodic table and the rare earth metals stretched from Cerium (Ce) to Lutetium (Lu). The other rare earth metals are situated in ...
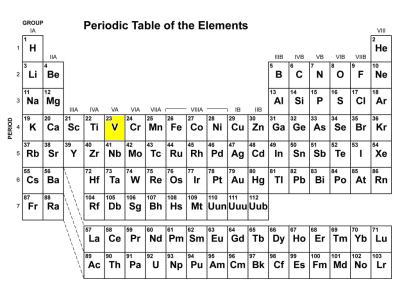
What are transition metals and where are they located?
The main group elements include the active metals in the two columns on the extreme left of the periodic table and the metals, semimetals, and nonmetals in the six columns on the far right. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table.
Why are transition metals in the middle of the periodic table?
The transition metals are located in the middle of the periodic table in between the elements of the left and the right. They act as a bridge or transition between the two sides of the periodic table.
Where are transition metals found on the periodic table GCSE?
The transition metals are placed in the centre of the periodic table, between groups 2 and 3. They are generally hard and dense, and less reactive than the alkali metals.
How many transition metals are in the periodic table?
38 elementsMost scientists simply regard the transition metals as the elements in the d-block (groups 3-12) on the periodic table. There are total of 38 elements in this group including Cobalt, Nickel, Iron, Rhodium, Gold, Silver, Cooper, Scandium, Titanium, Vanadium, Manganese, Zinc and Mercury.
How do you know if an element is a transition metal?
Many scientists describe a "transition metal" as any element in the d-block of the periodic table, which includes groups 3 to 12 on the periodic table. In actual practice, the f-block lanthanide and actinide series are also considered transition metals and are called "inner transition metals".
What are transition metals GCSE?
Most metals are transition metals . They include iron and other metals used in construction, gold and other precious metals. The transition metals are placed in the central part of the periodic table . The transition metals are placed between groups 2 and 3 in the periodic table.
Which block has transition elements?
d-block elementsd-block elements are known as transition elements.
What do group 1 and transition metals have in common?
Transition metals, on the other hand, are d block elements, but not all d block elements are transition metals. Group 1 metals and transition metals are similar in the fact that both have unpaired electrons.
How are elements in the middle of the table classified state a conclusion?
The majority of elements in the periodic table are classified as metals. The transition metals lie in the center of the table, spanning groups three through 12. These elements are solid at room temperature, except mercury, and have the metallic color and malleability expected of metals.
What are the inner transition metals in the periodic table?
The period 6 inner transition metals (lanthanides) are cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu).
Why are elements in group 3 to 12 called transition metals?
Elements with partially filled d orbitals are known as transition elements or transition metals. An element with a partly full d subshell or the ability to create stable cations with an incompletely filled d orbital is defined as a transition element by IUPAC.
Why inner transition elements are separated?
The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. The reason for this placement has to do with the electron configurations of these elements.
Where are inner transition metals located?
Inner transition metals are located in the two rows at the bottom of the periodic table.
Why Inner Transition Elements are called so?
Because they have similar properties like transition elements, and they are placed in the inner section as an extension of group 3.
What happens if the inner transition elements are not placed in the bottom?
If the inner transition elements were not placed in the bottom, then there will be a longer periodic table like this.
What metals are used to make nuclear weapons?
Uranium and plutonium are inner transition metals which are used for manufacturing nuclear weapons.
Which elements are placed separately in the two rows at the bottom of the periodic table?
They have their valence electrons in the f-orbitals. Hence, the transition metals (lanthanoids and actinoids) are placed separately in the two rows at the bottom of the Periodic table. And as inner transition elements have valence electrons in f-orbitals, ...
What metals are used to make magnets?
Neodymium (Nd), Cerium (Cm) and Samarium (Sm) are mixed with other metals to prepare strong magnets.
Where are lanthanides found?
The lanthanides are found naturally from the earth crust but they are found from very rare locations. Most actinides elements are artificially prepared in laboratory and they are radioactive in nature. The inner transition elements have the valence electrons in the f-orbitals.
Tips & Thanks
Posted 7 years ago. Direct link to Posts Only's post “Why does Zinc shed electr...”
Video transcript
In the last video, we saw the classification of elements into groups on the periodic table, and we stopped with the definition for a transition metal. There are two ways to think about transition metals.
Where are metals located on the periodic table?
The metals are located on the left side of the Periodic Table.
What are metals in Periodic table?
Metals are the elements which have the tendency to donate or lose electrons to form positive ions.
How many electrons do metals lose in a chemical reaction?
The atoms or metals have generally 1, 2 or 3 electrons in the outermost orbit, and they lose these electrons during a chemical reaction.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Which group of metals is the most reactive?
They are the Alkali metals of group 1. In 1st group, as we move down from top to bottom, the reactive of metals increases. Thus the bottom most element of group 1 (i.e francium) is the most reactive metal on the Periodic table. ( Note: Francium is a laboratory made element.
Why do metals make a ringing sound?
Metals produce ringing sound when they are stuck hard. This indicates that metals are sonorous in nature.
What are the elements in group 3 to group 12?
The elements lying in group 3 to group 12 are known as Transition metals (or transition elements). Transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14. Thus these metals are known as “Transition metals”.
Why are transition metals called transition metals?
These elements are called " transition metals " because the electrons of their atoms make the transition to filling the d subshell or d sublevel orbital. Thus, the transition metals are also known as the d-block elements. Here is a list of elements that are considered to be transition metals or transition elements.
Which transition metals have high melting points?
The transition metals, as a group, have high melting points. The exception is mercury, which is a liquid at room temperature. By extension, these elements also have high boiling points. Their d orbitals become progressively filled as you move from left to right across the periodic table.
Why do transition metals have colored complexes?
Transition metals form colored complexes, so their compounds and solutions may be colorful. The complexes split the d orbital into two energy sublevels so that they absorb specific wavelengths of light. Because of the different oxidation states, it's possible for one element to produce complexes and solutions in a wide range of colors.
What is the largest group of elements on the periodic table?
The largest group of elements on the periodic table is that of the transition metals, which is found in the middle of the table. Also, the two rows of elements below the main body of the periodic table (the lanthanides and actinides) are special subsets of these metals. These elements are called " transition metals " because the electrons ...
What is the oxidation state of iron?
For example, iron commonly carries a 3+ or 2+ oxidation state. Copper may have a 1+ or 2+ oxidation state. The positive oxidation state means the transition metals typically form ionic or partially ionic compounds. Atoms of these elements have low ionization energies.
Is transition metal a conductor of heat?
They are excellent conductors of heat and electricity. The transition metals are malleable (easily hammered into shape or bent). These metals tend to be very hard. Transition metals look shiny and metallic.
Is a transition metal reactive?
Although the transition metals are reactive, they are not as reactive as elements belonging to the alkali metals group. Many transition metals form paramagnetic compounds. Helmenstine, Anne Marie, Ph.D. "Transition Metals: List and Properties.".
What are the elements that are used in transitional metals?
Many of the elements are technologically important: titanium, iron, nickel, and copper, for example, are used structurally and in electrical technology. Second, the transition metals form many useful alloys, with one another and with other metallic elements.
How to understand transition metals?
The relative locations of the transition metals in the periodic table and their chemical and physical properties can best be understood by considering their electronic structures and the way in which those structures vary as atomic numbers increase.
What is the electron configuration of zinc?
Through the next nine elements, in increasing order of atomic number, electrons are added to the 3 d orbitals until, at the element zinc, they are entirely filled and the electron configuration is [Ar]3 d10 4 s2.
How to understand electron configurations?
To understand the electron configurations of other atoms, it is customary to employ the Aufbau (German: “building up”) principle, the basis of which is that, to achieve a multi-electron configuration, the required number of electrons must be added to the orbitals one at a time, filling the most stable orbitals first, until the total number has been added. Thus, in “building up” the periodic table, one progresses from one element to the next by adding one proton to the nucleus and one electron to the atomic region outside the nucleus. There is one restriction upon this conceptualization, namely, the Pauli exclusion principle, which states that only two electrons may occupy each orbital. Thus there can be no more than two electrons in any s orbital, six electrons in any set of p orbitals, ten electrons in any set of d orbitals, etc. In carrying out this process, however, one cannot simply use the ordering of electron orbitals that is appropriate to the hydrogen atom. As electrons are added they interact with each other as well as with the nucleus, and as a result the presence of electrons in some orbital causes the energy of an electron entering another orbital to be different from what it would be if this electron were present alone. The overall result of these interelectronic interactions (sometimes referred to as shielding) is that the relative order of the various atomic orbitals is different in many-electron atoms from that in the hydrogen atom; in fact, it changes continuously as the number of electrons increases.
What is the electronic structure of an atom?
Thus, by electronic structure, or configuration, of an atom is meant the way in which the electrons surrounding the nucleus occupy the various atomic orbitals available to them. The simplest configuration is the set of one-electron orbitals of the hydrogen atom.
What is the first element in the inner transition?
2,856. The first of the inner transition series includes the elements from cerium (symbol Ce, atomic number 58) to lutetium (symbol Lu, atomic number 71). These elements are called the lanthanoids (or lanthanides) because the chemistry of each closely resembles that of lanthanum.
How are orbitals classified?
The orbitals can be classified, first, by principal quantum number, and the orbitals have increasing energy as the principal quantum number increases from 1 to 2, 3, 4, etc. (The sets of orbitals defined by the principal quantum numbers 1, 2, 3, 4, etc., are often referred to as shells designated K, L, M, N, etc.)
What are the transition metals in the periodic table?
The elements in the periodic table are often divided into four categories: (1) main group elements, (2) transition metals, (3) lanthanides, and (4) actinides. The main group elements include the active metals in the two columns on the extreme left of the periodic table and the metals, semimetals, ...
What are the transition metals?
The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. The lanthanides and the actinides at the bottom of the table are sometimes known as the inner transition metals because they have atomic numbers that fall between the first and second elements in the last two rows ...
What is the difference between transition metals and main group metals?
The transition metals are more electronegative than the main group metals, for example, and are therefore more likely to form covalent compounds. Another difference between the main group metals and transition metals can be seen in the formulas of the compounds they form. The main group metals tend to form salts (such as NaCl, Mg 3 N 2, ...
What happens when manganese is oxidized?
When the manganese atom is oxidized, it becomes more electronegative. In the +7 oxidation state, this atom is electronegative enough to react with water to form a covalent oxide, MnO 4-.
How many oxidation states are there in transition metals?
Most transition metals form more than one oxidation state.
Why are oxidation states common?
Some of these oxidation states are common because they are relatively stable. Others describe compounds that are not necessarily stable but which react slowly. Still others are common only from a historic perspective. Common Oxidation States of the First Series of Transition Metals.
When are electrons removed from the valence shell?
In general, electrons are removed from the valence-shell s orbitals before they are removed from valence d orbitals when transition metals are ionized.
Where are transition metals found?
Transition metals are found in the middle part of the Periodic table (from Group 3 to group 11).
What is the inner transition metal?
The answer is: These elements have somewhat similar properties like that of transition metals, plus they are the elements of group 3 only, but they are placed at the bottom of the Periodic table as the inner section of group 3. Hence they are known as inner transition metals.
How many electrons are in an alkaline metal?
All the alkaline earth metals have 2 electrons in their outermost orbit.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Where are alkaline earth metals located?
Alkaline earth metals are located on the left side of the Periodic table in group 2. Alkaline earth metals are also the reactive type of metals but they are less reactive as compared to alkali metals. All the alkaline earth metals have 2 electrons in their outermost orbit.
Which metal is the most reactive?
Alkali metals are the most reactive type of metals from the entire Periodic table of elements. As we move down the group from top to bottom in the group 1, the reactivity of alkali metals increases. List of alkali metals with atomic number, symbol and name. Atomic number. Symbol.
What color is heavy metal?
These heavy metals are displayed on the Periodic table with red color (see above image)
