What are the metals and nonmetals in the periodic table?
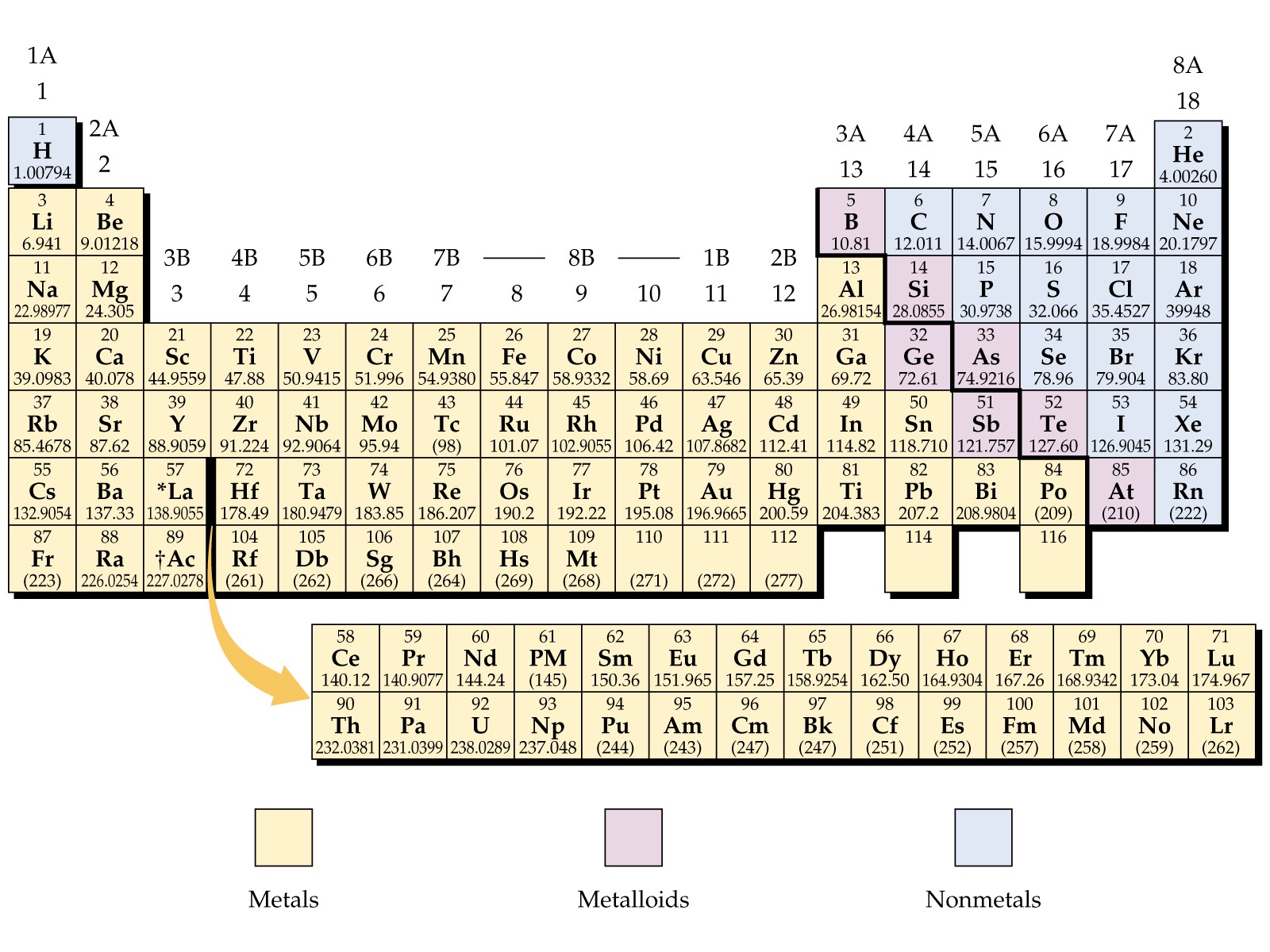
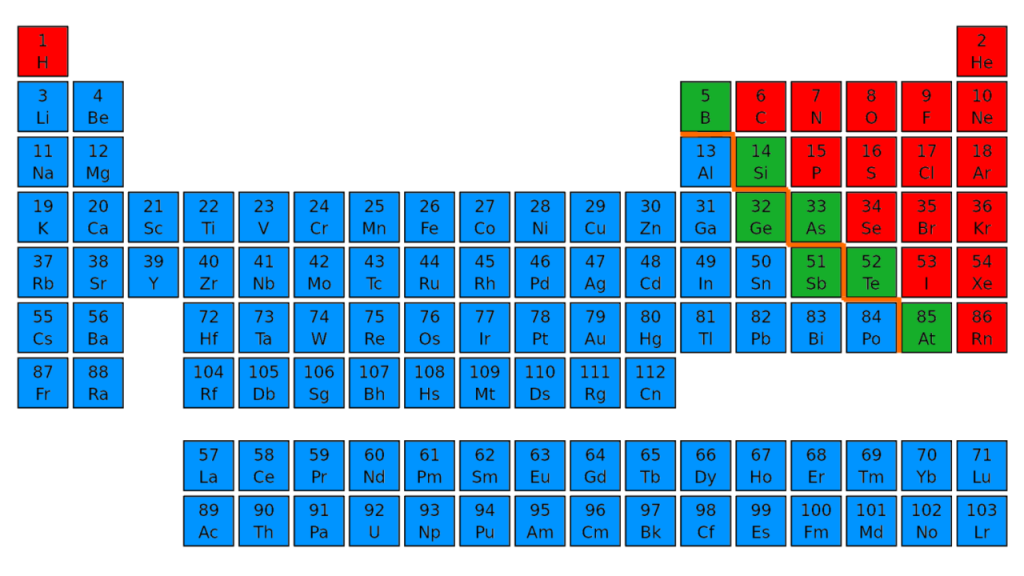
Metals are located on the left of the periodic table, and nonmetals are located on the upper right. They are separated by a diagonal band of semimetals. Metals are lustrous, good conductors of electricity, and readily shaped (they are ductile and malleable), whereas solid nonmetals are generally brittle and poor electrical conductors.
What is the most interesting element on the periodic table?
What Is the Coolest Element?
- Carbon. Carbon is cool for several reasons. ...
- Sulfur. You usually think of sulfur as a yellow rock or powder, but one of the cool things about this element is that it changes color under different conditions.
- Lithium. All of the alkali metals react spectacularly in water, so why did lithium make the list while cesium did not?
- Gallium. ...
What are the 22 non metals?
What are the twenty two non-metals? The nonmetal element group consists of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur and selenium. The elements at the top of the group are gases, but they become liquids and solids moving down the group. The halogens are fluorine, chlorine, bromine, iodine, and astatine.
What is a nonmetal on the periodic table?
The nonmetals or non-metals are a group of elements located on the right side of the periodic table (except for hydrogen, which is on the top left). These elements are distinctive in that they typically have low melting and boiling points, don't conduct heat or electricity very well, and tend to have high ionization energies and electronegativity values.

Where are transition metals located in the periodic table?
June 10, 2021 September 14, 2020 by Admin. The transition metals are located in the middle of the Periodic table from group 3 to group 11. From the above image, you can easily see where are Transition Metals located on the Periodic Table. According to some chemists, the d-block elements are known as transition elements.
How many transition metals are there in the periodic table?
There are 31 commonly known transition metals on the periodic table as shown in the above image by yellow color. (According to the definition given by IUPAC)
What is the transition from metallic to nonmetallic?
In other words, the transition of metallic nature to nonmetallic nature appears in these elements. As the properties of these elements show transition from electropositive nature to electronegative nature, they are called transition metals. Definition by IUPAC. Transition elements (or transition metals) are those elements which have partially ...
Why are transition elements called transition elements?
Here is a reason: Transition elements are called so because their properties show the transition from electropositive s-block elements to the electronegative p-block elements. These elements form a bridge between the best metals and the best nonmetals. (Metals are on the left side of periodic table and nonmetals are on the right side ...
Why are group 12 elements not considered transition metals?
According to the definition given by IUPAC, the group 12 elements (Zn, Cd and Hg) are not considered as transition metals because they do not have incomplete d-orbitals either in their elemental state (Zn, Cd, Hg) or oxidation state (Zn 2+, Cd 2+, Hg 2+ ). Read: The exact reason why group 12 elements are not included as transition elements? (Explained with examples)
Where are transition metals located?
Well, transition metals are located in the middle of the Periodic table from group 3 to group 11.
Which group of elements are transition metals?
Finally as a summary, simply remember that the elements lying from group 3 to 11 are the transition metals on the periodic table.
Which group of periodic table is alkali metals?
Alkali metals are present in the first group of Periodic table. First group indicates that they have only one electron in their outermost orbit. As only one electron is present in the outermost orbit, it is very easy to lose or donate this electron during a chemical reaction.
Where are the symlinks on the periodic table?
They are on the left side of the Periodic table (in group 1).
Why are alkali metals so reactive?
There are many reasons behind the reactivity of alkali metals. Let us discuss them one by one.
What metals react with water?
When these metals (Li, Na, K, Rb, Cs, Fr) react with water, they form alkalis (i.e strong base).
How many electrons are in an alkali metal?
Look, as we move down the group from top to bottom, the atomic size increases. Also alkali metals have only one electron in their outermost orbit.
Which element is the most reactive?
And hence the element with bigger atomic size (i.e Francium) is most reactive alkali metal. ( Note: Francium is a laboratory made element. It is available in a very less quantity. And hence for any practical purposes, cesium is considered as the most reactive metal in Periodic table.
Where are alkali metals located?
Alkali metals are located in group 1 on the left side of the Periodic table.
Where are transition metals found?
Transition metals are found in the middle part of the Periodic table (from Group 3 to group 11).
Where are alkaline earth metals located?
Alkaline earth metals are located on the left side of the Periodic table in group 2. Alkaline earth metals are also the reactive type of metals but they are less reactive as compared to alkali metals. All the alkaline earth metals have 2 electrons in their outermost orbit.
How many electrons are in an alkaline metal?
All the alkaline earth metals have 2 electrons in their outermost orbit.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
What is the inner transition metal?
The answer is: These elements have somewhat similar properties like that of transition metals, plus they are the elements of group 3 only, but they are placed at the bottom of the Periodic table as the inner section of group 3. Hence they are known as inner transition metals.
Which metal is the most reactive?
Alkali metals are the most reactive type of metals from the entire Periodic table of elements. As we move down the group from top to bottom in the group 1, the reactivity of alkali metals increases. List of alkali metals with atomic number, symbol and name. Atomic number. Symbol.
What color is heavy metal?
These heavy metals are displayed on the Periodic table with red color (see above image)
Where are metalloids found in the periodic table?
Also we can say that metalloids are present in the diagonal region of the p block on Periodic table.
What is the color of the metalloids in the periodic table?
June 10, 2021 August 25, 2020 by Admin. Metalloids are located between the metals and nonmetals. The orange color on the Periodic table represents metalloids . They form a separating boundary between the metals and nonmetals. In other words, metalloids (semimetals) are located on the right side of the post transition metals and on the left side ...
What are the properties of metalloids?
Let us discuss the physical properties as well as chemical properties of metalloids/semimetals.
What is the chemical reaction between halogens and metalloids?
Metalloids + Halogens = Compounds (metalloid s elements reacts with halogens and finally compounds are formed by this chemical reaction) Metalloids have different metallic allotropes as well as nonmetallic allotropes. Metalloids have the property to form glasses on oxidation and so that are used in glass manufacturing.
What are elements that show some properties of metals as well as solid nonmetals called?
The elements that show some properties of metals as well as solid nonmetals are called metalloids . Metalloids look like metals, but they are not. Metalloids are brittle like solid nonmetals. Metalloids are neither conductor nor insulated. Source ( James L Marshall, Silicon, Germanium / CC BY-SA, CC BY ) The examples of metalloids are:
Why are metalloids called semiconductors?
Metalloids are called semiconductors because they are not good conductors like metals and also they are not bad conductors like nonmetals. They have the conductivity which is higher than nonmetals, but lower than metals. Hence metalloids are known as semiconductors.
How many metalloids are there?
Hence, there are total 6 known metalloids/semimetals on the Periodic table.
Where is hydrogen on the periodic table?
Exception: Hydrogen is a nonmetal which is located on the left top corner of the Periodic table.
How many nonmetals are there in the periodic table?
There are 18 nonmetals on the Periodic table. All these nonmetals are located on the upper right corner of the Periodic table (Hydrogen is located on the left top corner) In the above image, the nonmetals are represented in yellow color. [ Note: Astatine (atomic number 85) shows characteristics of nonmetals (halogens) as well as metalloids.
What are nonmetals?
Or in other words nonmetals are those elements which do not possess the properties of metals.
How many electrons are in the outermost orbit of a nonmetal?
Nonmetals have 4 to 8 electrons in their outermost orbit. (Halogens have 7 electrons and noble gases have 8 electrons in outermost orbit)
Why are nonmetals oxidizing agents?
Nonmetals are oxidizing agents because they gain electron/s during a chemical reaction and get reduced.
What does it mean when a solid nonmetal breaks?
Solid nonmetals are brittle in nature. That means they break easily when force is applied on them.
Which nonmetals are the most reactive?
Actually the halogens are the most reactive nonmetals, but we know that as we move down the group, the electronegativity decreases. In other words, Fluorine is at the top of the halogen group and it has less atomic size plus it needs only one electron to complete the octet.