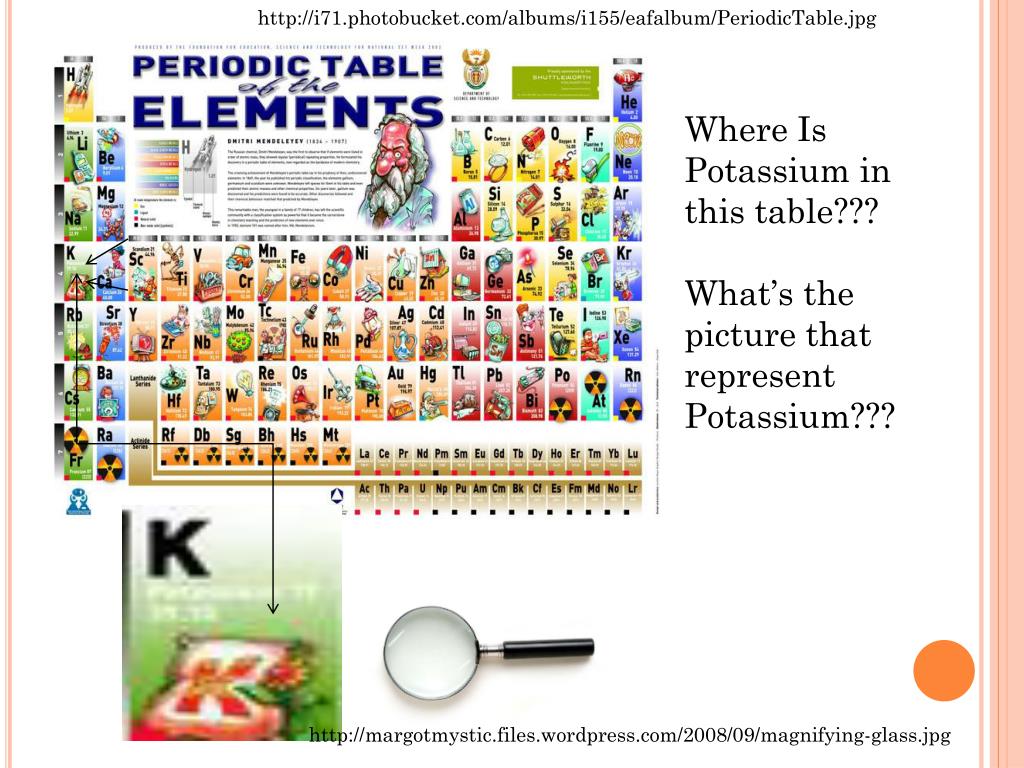
Where is potassium K located on the periodic table?
Group 1potassium (K), chemical element of Group 1 (Ia) of the periodic table, the alkali metal group, indispensable for both plant and animal life.
What group and period is potassium in?
Fact boxGroup163.5°C, 146.3°F, 336.7 KPeriod4759°C, 1398°F, 1032 KBlocks0.89Atomic number1939.098State at 20°CSolid39K2 more rows
Why is potassium placed in Period 4?
Solution : The electronic configuration of potassium is (2, 8, 8, 1) . It has four shells so it belongs to the period 4 . The period 4 has elements with the 4th shell being filled. Potassium has 1 electron in its valence shell.
Is potassium a nonmetal?
Potassium is a soft, silvery-white metal, member of the alkali group of the periodic chart.
What type of element is potassium?
alkali metalPotassium is a chemical element with symbol K and atomic number 19. Classified as an alkali metal, Potassium is a solid at room temperature.
What element is in Group 4 period 5?
ZirconiumThe symbol of the element in Group 4 and Period 5 is Zr. It is the symbol of Zirconium. Was this answer helpful?
What charge is potassium?
+11.17: IonsElementProtonsNet ChargePotassium atom190Potassium ion19+1Sulfur atom160Sulfur ion16−2Aug 24, 2020
Why is potassium called K?
The word potassium stems from the English “pot ash,” which was used to isolate potassium salts. We get K from the name kalium, given by the German chemist Martin Heinrich Klaproth, which stemmed from alkali, which stemmed from the Arabic al-qalyah, or “plant ashes.”
Is potassium a main group element?
The main group elements are classified as belonging to the s- and p-blocks in the periodic table. They range from gases such as fluorine and oxygen through nonmetals (e.g., boron, phosphorus, and sulfur) and semimetals (metalloids; e.g., germanium) to very reactive metals such as sodium and potassium.
Why is potassium K on the periodic table?
The word potassium stems from the English “pot ash,” which was used to isolate potassium salts. We get K from the name kalium, given by the German chemist Martin Heinrich Klaproth, which stemmed from alkali, which stemmed from the Arabic al-qalyah, or “plant ashes.”
Why is potassium a metal?
Most potassium occurs in the Earth's crust as minerals, such as feldspars and clays. Minerals mined for their potassium are pinkish and sylvite, carnallite and alunite. Hence, potassium is a metal.
What charge is potassium?
+11.17: IonsElementProtonsNet ChargePotassium atom190Potassium ion19+1Sulfur atom160Sulfur ion16−2Aug 24, 2020
Where is potassium on the periodic table?
Potassium is the 19 th element on the periodic table. It is located in period 4 and group 1. In other words, it is the element in the first column of the table that begins the fourth row.
What is the atomic number of potassium?
Potassium is an alkali metal, like sodium, cesium, and the other elements in group 1. It is the 7th most abundant element in the Earth's crust. It has atomic number 19, element symbol K, and an atomic weight of 39.0983. Helmenstine, Anne Marie, Ph.D. "Where Is Potassium Found On The Periodic Table?".
Where is potassium found in the world?
Mining extracts about 35 million tonnes a year. Most potassium minerals are found in igneous rocks and are sparingly soluble.
How much potassium is in a human body?
The average human consumes up to 7 grams of potassium a day, and stores about 140 grams in the body cells. A normal healthy diet contains enough potassium, but some foods such as instant coffee, sardines, nuts, raisins, potatoes and chocolate have above average potassium content.
How is potash made?
The crude potash can be made more caustic or 'pure' by treating a solution of it with lime water, calcium hydroxide. The potassium carbonate and calcium hydroxide solutions react with a bit of chemical partner-swapping: insoluble calcium carbonate or chalk precipitates out, leaving a solution of potassium hydroxide. It was from this pure hydroxide that Davy first isolated the metal potassium. To do this he used the relatively new force of electricity.
What element is named after a cooking utensil?
Potassium - the only element named after a cooking utensil. It was named in 1807 by Humphry Davy after the compound from which he isolated the metal, potash, or potassium hydroxide.
What is potassium carbonate?
A soft, silvery metal that tarnishes in air within minutes. The greatest demand for potassium compounds is in fertilisers. Many other potassium salts are of great importance, including the nitrate, carbonate, chloride, bromide, cyanide and sulfate. Potassium carbonate is used in the manufacture of glass.
What are the minerals that make up metal?
There are, however, other minerals such as sylvite (potassium chloride), sylvinite (a mixture of potassium and sodium chloride) and carnallite (potassium magnesium chloride) that are found in deposits formed by evaporation of old seas or lakes.
Why is potassium important?
Potassium is essential to life. Potassium ions are found in all cells. It is important for maintaining fluid and electrolyte balance.
Where does potassium come from?
It was first obtained in pure form by Humphry Davy (1807), through the process of electrolysis. The symbol of potassium is K and is derived from kali (alkali), which is derived from Arabic work for plant ashes ( al-qalyah) [1].
What is potassium in?
It is a major component of variety of proteins and enzymes. Potassium is also present in fruits (high concentrations in bananas, avocadoes), vegetables (potatoes, tomatoes, beet green, white beans), fish and meat.
What is the name of the element that was discovered in 1807?
Potassium. Potassium is an alkali metal and was obtained in pure elemental form in 1807 by Hymphry Davy. It is a biologically essential element and also has various industrial applications, including fertilizers, pesticides, explosives etc.
What is the use of potassium carbonate?
Potassium carbonate, usually termed as potash is widely used in the manufacturing of soap, glass, fluorescent lamps, fire extinguishers, and color TV.
How was potassium discovered?
Potassium was discovered from the ashes of plants and its name was derived from potash (plant ashes). In early days, potassium was extracted by placing ashes of burnt tree in a pot along with water. the mixture was heated and the solution by evaporated to obtain potash (potassium). It was first obtained in pure form by Humphry Davy (1807), through the process of electrolysis. The symbol of potassium is K and is derived from kali (alkali), which is derived from Arabic work for plant ashes ( al-qalyah) [1].
What is potassium chromate used for?
Potassium is used in leather tanning industries. It is used to make fireworks, matches and explosives (Potassium chlorate).
How much potassium is needed for a healthy body?
Potassium is an essential nutrient and human body requires a balanced supply (about 3.3 gram daily) of potassium. Deficiency of potassium can lead to hypokalemia and hypertension [4]. The reaction of potassium with water is highly exothermic and splashes can lead to skin burns and damage.
How many protons does potassium have?
Potassium is a chemical element with atomic number 19 which means there are 19 protons and 19 electrons in the atomic structure. The chemical symbol for Potassium is K.
Which element has the same electron configuration in the outer electron shell?
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How many protons does silicon have?
Silicon is a chemical element with atomic number 14 which means there are 14 protons and 14 electrons in the atomic structure. The chemical symbol for Silicon is Si.
What is the charge of an atom?
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
How are atoms determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
What is the most common type of boron?
There are over 100 different borate minerals, but the most common are: borax , kernite, ulexite etc. Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B.
What is the melting point of potassium?
Potassium Properties: Potassium's melting point is 63.25°C, boiling point is 760°C, specific gravity is 0.862 (20°C), with a valence of 1. Potassium is one of the most reactive and electropositive of metals.
What is the use of potassium and sodium?
Uses: Potash is in high demand as a fertilizer. Potassium, found in most soils, is an element that is essential for plant growth. An alloy of potassium and sodium is used as a heat transfer medium.
Where is potash mined?
In addition to other locations, potash is mined in Germany, Utah, California, and New Mexico.
Is potassium salt free?
Sources: Potassium is the 7th most abundant element on earth, making up 2.4% of the earth's crust, by weight. Potassium is not found free in nature. Potassium was the first metal isolated by electrolysis (Davy, 1807, from caustic potash KOH).
What is the symbol for potassium?
Potassium, represented with the symbol K , is one of the alkali metals. This element is soft and has a silvery-white color. Just like other alkali metals, potassium mainly takes the form of salts. Additionally, potassium ions are essential for normal nerve transmission inside of living cells.
How many isotopes of potassium are there?
There are three major isotopes of potassium: 39 K, 40 K, and 41 K. Among them, only 40 K is radioactive. In general, a radioactive isotope is one whose nuclei are unstable and can give off energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays.
What is the purpose of potassium salts in fertilizer?
Fertilizers use potassium salts as a source of plant nutrition.
Why is potassium important for the nervous system?
In the nervous system, potassium in its cationic form helps to send messages between the brain and body. These messages are nerve impulses that control heartbeat, muscle contractions, and other body functions. Potassium moving out of the cell, and sodium into the cell creates a change in cell voltage, which in turn creates these nerve impulses. Therefore, getting enough potassium is essential to maintain a healthy nerve function.
Where do potassium salts come from?
Potassium salts reside in evaporite deposits in ancient lake bottoms and seabeds. The extraction of potassium salts from these extensive sources is economically friendly. The basic potassium source types are the carnallites, containing 45-85% carnallite and 18-50% halite with small amounts of sylvite, anhydrite, clay minerals, and carbonates; sylvinite including 95–98% sylvite and halite, the remainder being an insoluble residue; and hard salt, containing 8–25% sylvite, 18–30% kieserite, 40–60% halite, and 0.5–2.0% carbonates, anhydrite, and clay minerals. The majority of the known resources are concentrated in the USSR in the Urals (Solikamsk, Perm Oblast), western Kazakhstan, western Ukraine, and Byelorussia. Significant foreign deposits include those in the German Democratic Republic (Stassfurt), the Federal Republic of Germany (Hanover, the Harz, Hesse, Baden), the USA (the Carlsbad region in New Mexico; Lake Searles in California), Canada (Saskatchewan), France (Alsace), and Italy (Sicily).
How to increase potassium levels?
Alternatively, potassium deficiency leads to symptoms such as leg cramps, paralysis of muscles, abnormal heartbeat, etc. For those in the low-to-healthy range of potassium, research found that increasing potassium levels will have a significant effect on lowering population blood pressure. In addition, an increase in potassium intake may have direct beneficial effects on the cardiovascular system. The best way of increasing potassium intake is to increase the consumption of fruit and vegetables, which also has beneficial effects on health independent of potassium intake.
Why do stores sell potassium?
In addition, because potassium is essential for human health, most general stores sell potassium for people who do not have enough potas sium in their daily diet or have potassium deficiency because of illness.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the chemical symbol for potassium?
On the periodic table, the chemical symbol for potassium is the letter K . Thus, people sometimes confuse potassium with vitamin K.
What is the most common type of vitamin K?
Vitamin K1 — also known as phylloquinone — is the type usually found in leafy green vegetables. It’s also the most common type of vitamin K in the human diet ( 5. Trusted Source. ). Vitamin K2 is a group of compounds known as menaquinones. They’re often present in animal products and fermented foods.
What is the purpose of Vitamin K?
Vitamin K is a group of fat-soluble vitamins the body needs to produce proteins for blood clotting and bone production, among other functions ( 5#N#Trusted Source#N#, 6#N#Trusted Source#N#, 7#N#Trusted Source#N#).
Is potassium a micronutrient?
Regularly consuming foods that are rich sources of vitamin K and potassium can contribute to an overall healthy diet. These micronutrients each have benefits of their own, and they may even share a few similar benefits. For example, scientists have studied both for their potential effects on bone and heart health ( 17.
Is vitamin K2 bioavailable?
food, supplements, and in some foods as an additive. Bioavailability. vitamin K2 may be more bioavailable than K1. still unclear which forms the body absorbs best. Uses. blood clotting and bone metabolism.
Is potassium the same as vitamin K?
Though vitamin K and potassium are both important, they’re not the same type of compound.
Does potassium help with osteoporosis?
Like vitamin K, potassium may help bone health in postmenopausal women. A recent study found potassium may have more pronounced effects on bone health and osteoporosis risk in that population ( 35 ).

Occurrence
Physical Characteristics
- Potassium is silvery-white, soft metal with second least density (0.89 g/cm3). At standard conditions, potassium can be cut with a knife. Potassium undergoes tarnishing when exposed to air and oxygen. Potassium can float on water. It has a boiling point of 770 °C and melting point of 63 °C. It belongs to Group 1 (1A) of periodic table. Potassium gi...
Chemical Characteristics
- Potassium has one valence electron, that is readily lost to acquire a positive charge. Potassium is one of the alkali metals and it can combine with anions to from salts. It is very reactive and is rapidly oxidized when exposed to air forming potassium peroxide. Potassium gives a vigorous reaction with water (forming potassium hydroxide) and burns with a pale violet color. Potassiu…
Significance and Uses
- It is widely used in fertilizers. It is the largest industrial usage of potassium and about 90% of potassium produced in the world is utilized in the production of various fertilizers.
- It is a commonly used food additive and is main component of baking powder and silvering mirrors.
- Potassium chromate is a widely used pigment and is part of dyes, inks, stains etc.
- It is widely used in fertilizers. It is the largest industrial usage of potassium and about 90% of potassium produced in the world is utilized in the production of various fertilizers.
- It is a commonly used food additive and is main component of baking powder and silvering mirrors.
- Potassium chromate is a widely used pigment and is part of dyes, inks, stains etc.
- Potassium is used in leather tanning industries.
Health Hazards
- Potassium is an essential nutrient and human body requires a balanced supply (about 3.3 gram daily) of potassium. Deficiency of potassium can lead to hypokalemia and hypertension . The reaction of potassium with water is highly exothermic and splashes can lead to skin burns and damage. It reacts explosively with sulphur acid, so special care is required during handling of po…
Isotopes of Potassium
- There are about two dozen isotopes of potassium, and three of them occur naturally (39K, 40K and 41K). 40K is the radioactive isotope. It is present in human body in great abundance and around 4,400 nuclei of 40K decay per second occur in an average human body. 40K provides the largest source of natural radioactivity and used widely for rock dating. 39K is the most common …