
Where are the solids located on the periodic table?
Located to the left of the Periodic Table. Metalloids. Located along the zigzag line of the Periodic Table ... Nonmetals. gain electrons to form negative ions. Metals. Solid may have luster, and is brittle. Metals. Solids are malleable. Metals. Tends to lose electrons and form positive ions ... investigating the arrangement of the periodic ...
Where is magnesium located on the periodic table?
Magnesium belongs to the Group 2 (IIA) of periodic tables which makes it one of the alkaline earth elements. The atomic number of magnesium is 12 and its atomic mass is 24.305. The density of magnesium is 1.738 grams per cubic centimeter.
Where is the bromine on the periodic table?
Bromine (Br) is a chemical element of the periodic table, located in the group 17 and the period 4, and has the atomic number 35. It is a reddish-brown liquid, whose name comes from the Greek word “bromos”, which means stench. It is a reactive nonmetal and is the third lightest halogen, after the chlorine element.
Where is gallium located on the periodic table?
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal at standard temperature and pressure. In its liquid state, it becomes silvery white.
How many protons and electrons are in hydrogen?
How are atomic nuclei determined?
What are the two forces that make up the nucleus?
What is the charge of an atom?
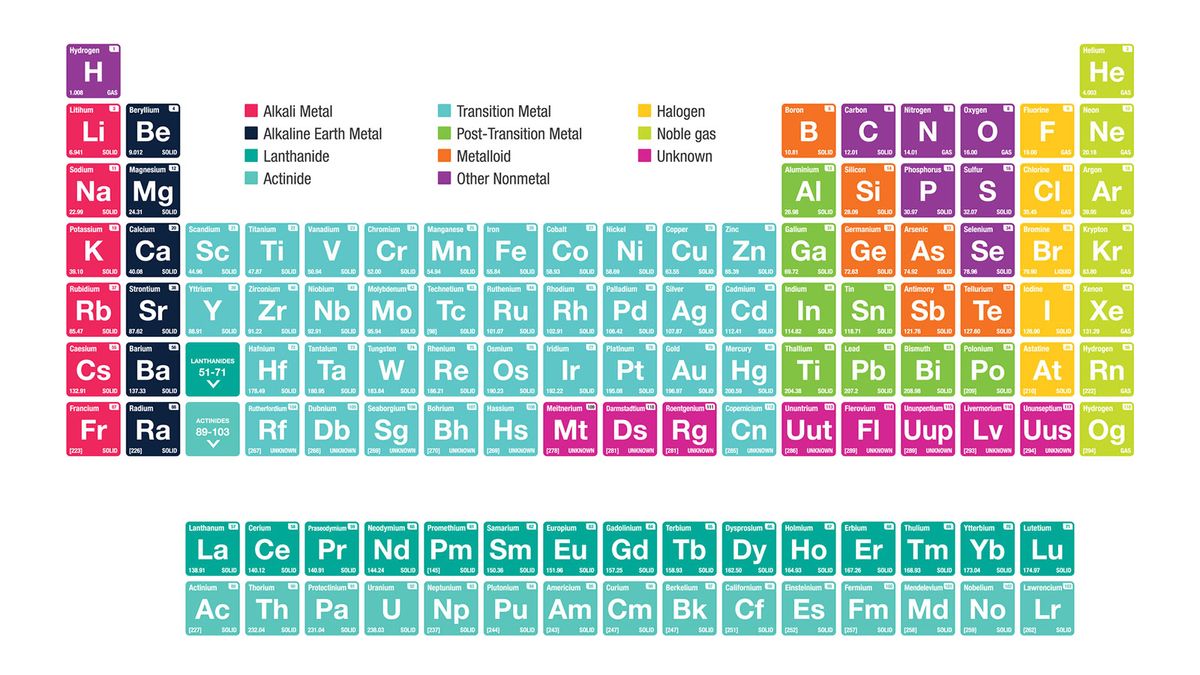
What is the periodic table?
How to determine the stability of an isotope?
What is the Pauli exclusion principle?
See 4 more
About this website
Chemical Symbol for Sodium - Na - Periodic Table
Chemical Symbol for Sodium. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure.The chemical symbol for Sodium is Na. Sodium is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table, because it has a single electron in its outer shell that it readily donates ...
The Salty Element Sodium | Periodic Table | ChemTalk
The Element Sodium Introduction to Sodium. The element sodium is a soft, flammable, silvery-white metal. Sodium, named after the English word ‘soda’, has compounds which are commonly used in our day-to-day lives.
Is Sodium a Metal or Nonmetal?
Yes, Sodium is a Metal. It’s a silvery white colored reactive metal. It is a soft metal and it can be cut even by using a kitchen knife. It is a light metal and it even floats on water.
What group is sodium in?
Sodium element is in group 1 and period 3 of the Periodic table. Sodium is the s-block element and it belongs to alkali metals group.
How many electrons does Na have?
This electron arrangement indicates that the outermost orbit of Sodium (Na) has 1 electron.
Why does the outermost electron escape during a chemical reaction?
And due to weak attractive force, the outermost electron gets easily escaped during a chemical reaction.
When sodium metal is heated with a flame, what happens to its electrons?
When sodium metal is heated with a flame, its outermost electron gets excited onto a higher energy level.
What color is sodium metal?
Sodium metal is silver white in color when it is freshly cut, but it suddenly forms an oxide layer if kept open in air.
Which element has less intermolecular force of attraction?
In short sodium element has less intermolecular force of attraction.
What is sodium hydroxide used for?
Sodium hydroxide can be used to remove sulfur from petrol and diesel, although the toxic soup of by-products that is formed has led to the process being outlawed in most countries. Sodium hydroxide is also used in biodiesel manufacture, and as a key component in products that remove blockages from drains.
What is the best salt to use for water softener?
Sodium carbonate (washing soda) is also a useful sodium salt. It is used as a water softener.
What is sodium salt used for?
The most common compound of sodium is sodium chloride (common salt). It is added to food and used to de-ice roads in winter. It is also used as a feedstock for the chemical industry. Sodium carbonate (washing soda) is also a useful sodium salt.
Where did the story of man and sodium come from?
Aside from being an essential nutrient, the story of man and sodium is said to begin all the way back in the time of the Pharaohs in Ancient Egypt, with the first recorded mention of a sodium compound in the form of hieroglyphics. It is difficult to describe a pictogram through speech but imagine a squiggly line over the top of a hollow eye-shape, over the top of a semicircle, with a left-facing vulture image next to them all. This pictogram meant divine or pure and its name is the root of the word natron, which was used to refer to washing soda, or sodium carbonate decahydrate, as we would know it today. Sodium carbonate was used in soap, and also, in the process of mummification thanks to its water absorbing and bacteria killing pH control properties.
Why is sodanum called sodanum?
In medieval Europe, however, sodium carbonate was also used as a cure for headaches, and so took the name sodanum, from the Arabic suda, meaning headache.
What color is the Bunsen burner?
Unfortunately, the intensity of the colour is such that if any of the compound is spilled into the Bunsen burner, it is cursed to burn with a blue and orange speckled flame seemingly forever. The reaction of sodium with water is a favourite demonstration, and clips of it abound on the internet.
What metal reacts with water?
Sodium is a soft metal that tarnishes within seconds of being exposed to the air. It also reacts vigorously with water.
How much sodium is needed for a healthy body?
Around 500mg of sodium is the minimum physiological requirement per day of human body. Sodium Chloride is daily used as seasoning and preservative in diet. In plants, sodium is a micronutrient which helps in metabolism. It is involved in synthesis of chlorophyll, opening and closing of stomata, and helps in uptake of water.
What is the reaction of sodium and carbon?
It is relatively less reactive with carbon, but at 625°C it reacts with carbon monoxide forming sodium carbide and sodium carbonate. With liquid ammonia sodium reacts to give blue colored solutions forming sodamide, but the reaction is quite slow. Organic acids also react with sodium to form sodium salts.
What is sodium made of?
Sodium in the form of salt (sodium chloride, NaCl) and soda (sodium carbonate, Na2CO3) have been known since prehistoric times [1] The name of element originated from Arabic word ‘suda’ which means headache, as the association of sodium with headache was known in early times. The metal was first isolated in 1807, through the electrolysis of sodium hydroxide by Sir Humphry Davy. In 1809, Ludwig Wilhelm, a German physicist and chemist, proposed the name Natronium for it. Its chemical abbreviation ‘Na’ was published in the system of atomic symbols in 1814 which is derived from its Latin name ‘natrium’ [2].
What is the importance of sodium in the cell?
Significance and Uses. Sodium is one of the most essential elements for life, as sodium and potassium keep a definite balance within the cell and are involved in maintaining an electrolyte balance across the cell membranes [6]. It helps in the regulation of blood pressure, blood volume, osmotic equilibrium and pH.
What are the characteristics of sodium hydroxide?
Chemical Characteristics. It is highly reactive in air and forms sodium hydroxide (NaOH), which is a strong base on reaction with water. In presence of air, sodium hydroxide film absorbs carbon dioxide, and lead to the formation of sodium bicarbonate. Sodium reacts with halogens under certain conditions to produce light.
How many isotopes of sodium are there?
Isotopes of Sodium. Twenty isotopes of sodium are identified, but only one, sodium-23 is stable. Two radioactive, cosmogenic isotopes which are the byproduct of cosmic ray spallation are known, Na-22 which has a half-life of 2.6 years and Na-24 with a half-life of 15 hours.
What is the atomic mass of sodium?
Sodium has an atomic number of 1 and atomic mass of 22.98. It is placed in group 1 of periodic table as it has a single electron in its outer most shell that it readily donates, creating a positively charged ion, the Na+ cation. At room temperature Sodium is soft, silvery-white metal which can be easily cut with a knife. It is highly reactive. In air it reacts with oxygen forming grayish white sodium oxide, so it is stored in an inert liquid such as kerosene or in nitrogen gas as it does not react with nitrogen. It is lighter than water. It is a good conductor of heat and electricity and exhibits the photoelectric effect i.e. the emission of electrons when exposed to light. Sodium and its compounds give yellow color to the flame which is the basic analytical test for sodium. It burns at a temperature more than 800 °C (1,500 °F) [5].
Where Can I Buy Elemental Sodium?
You can buy sodium on Amazon, Ebay or through online element stores like Luciteria. You can usually purchase it for around $0.50 – $1 USD per gram. It is usually sold stored under oil. If you’re looking for table salt, then you can buy it at your local grocery store.
How are sodium compounds used in the paper making process?
Firstly, they’re used to produce paper. In the paper-making process, sodium hydroxide treats/separates fibers in the pulling phase. Secondly, they’re used to produce clear glass. In this process, we heat sodium carbonate with calcium oxide. Thirdly, sodium compounds are used in creating different metals. For example, sodium combined with titanium tetrachloride produces titanium metal. In another reaction, sodium is a catalyst to create artificial rubber. Similarly, there are other materials (e.x. foodstuffs and fertilizer) which involve sodium in their production/usage.
Why does sodium float on water?
To begin with, sodium floats when placed on water. This is because sodium’s density is lower than that of water’s. Subsequently, an exothermic reaction will occur, and a solution of sodium hydroxide and hydrogen gas will form. This reaction releases so much heat that the sodium melts and releases the hydrogen from the added water. Further, this released hydrogen gas can catch fire.
What is the name of the element that is flammable?
The element sodium is a soft, flammable, silvery-white metal. Sodium , named after the English word ‘soda’, has compounds which are commonly used in our day-to-day lives. You might be familiar with table salt, which is NaCl (sodium chloride), a compound of sodium. It is one of the most reactive metals on the periodic table.
How is sodium made?
Sodium is commonly made through the vector process. Producing elemental sodium in a small scale laboratory is extremely difficult, though. However, it can be done with difficulty by performing the electrolysis of molten NaCl, but this is dangerous and it’s difficult to recover the sodium.
What is the Latin word for sodium carbonate?
Latin for sodium carbonate is ‘ natrium ’. So sodium’s elemental symbol ‘ Na ’ is derived from this word.
What is the electronegativity of sodium?
Sodium has an electronegativity of 0.93 (Pauling Scale). Sodium’s electron configuration is 1s22s22p63s1. Other elements in the alkali metals family include: potassium, lithium, cesium, and rubidium. Additionally, speaking of potassium, sodium and potassium’s chemical and physical properties are quite similar.
What is the process of producing sodium?
Most processes for the production of sodium involve the electrolysis of molten sodium chloride. Inexpensive and available in tank-car quantities, the element is used to produce gasoline additives, polymers such as nylon and synthetic rubber, pharmaceuticals, and a number of metals such as tantalum, titanium, and silicon.
What is the yellow color of a sodium lamp?
It is also widely used as a heat exchanger and in sodium-vapour lamps. The yellow colour of the sodium-vapour lamp and the sodium flame (the basis of an analytical test for sodium) is identified with two prominent lines in the yellow portion of the light spectrum. High-pressure sodium-vapour lamp bulb.
What are the compounds that contain sodium anion?
Compounds that contain the sodium anion, Na −, have also been synthesized. The principal commercial sodium compounds are the chloride, carbonate, and sulfate. The most important and familiar sodium compound is sodium chloride, or common salt, NaCl.
What are the uses of metallic sodium?
Significant uses. Two of the earliest uses of metallic sodium were in the manufacture of sodium cyanide and sodium peroxide. Significant quantities were used in the manufacture of tetraethyl lead as a gasoline additive, a market that disappeared with the advent of unleaded gasoline.
How many atoms of sodium are in a meteorite?
Analysis of meteorites indicates that the silicate material present has an average content of approximately 4.6 atoms of sodium for every 100 atoms of silicon. Sir Humphry Davy, detail of an oil painting after Sir Thomas Lawrence; in the National Portrait Gallery, London.
What is the name of the group of elements that are alkali metals?
Alternative Titles: Na, natrium. Sodium (Na), chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal. Sodium is the most common alkali metal and the sixth most abundant element on Earth, comprising 2.8 percent of Earth’s crust.
What is sodium in the periodic table?
Sodium (Na), chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal.
How many electrons does neon have?
Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. The chemical symbol for Neon is Ne.
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N.
How many protons does sodium have?
Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na.
How is atomic weight determined?
Therefore it is determined by the mass number (number of protons and neutrons).
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
What is the most abundant element in the Earth's crust?
Aluminium is a silvery-white, soft, nonmagnetic, ductile metal in the boron group. By mass, aluminium makes up about 8% of the Earth’s crust; it is the third most abundant element after oxygen and silicon and the most abundant metal in the crust, though it is less common in the mantle below.
What is the most common type of boron?
There are over 100 different borate minerals, but the most common are: borax , kernite, ulexite etc. Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B.
What is sodium ions?
Sodium ions are necessary in the depolarization of cells, vital to the neural system of most animals, including humans.
What is the range of chemical shifts?
There are only a small range of chemical shifts ~0.5eV and determination of chemical state may require use of corresponding regions to infer chemical state.
Can Na Auger peak be observed even if Na1s is not?
If sodium is buried (under carbon, for example), Na Auger peak may be observed even if Na1s is not (due to difference in electron kinetic energy).
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the CIAAW?
Since 1899 the IUPAC Commission on Isotopic Abundances and Atomic Weights ( CIAAW) has been evaluating atomic weights and abundances. For example, Carbon had an atomic weight of 12.00 in 1902 but today it is [12.0096, 12.0116]! Times sure have changed as the source of the sample will determine the value.
What is PubChem working with?
PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable.
Who is responsible for deciding what needs to be changed?
While much of what is in the periodic table is stable and unlikely to change, the IUPAC organization is responsible for deciding what needs to be changed. They have created criteria for what constitutes the discovery of a new element.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Did Mendeleev's predictions get dismissed?
There were plenty of skeptics and it took years to gain international acceptance, but once newly-discovered elements matched the ones that Mendeleev predicted, his patterns could not be dismissed. In addition, some of the properties that he "fudged" were later recalculated and found to be much closer to his predictions.
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
What are the two forces that make up the nucleus?
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
What is the charge of an atom?
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
What is the periodic table?
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements.
How to determine the stability of an isotope?
To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue. Only two stable nuclides have fewer neutrons than protons: hydrogen-1 and helium-3.
What is the Pauli exclusion principle?
It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. This fact has key implications for the building up of the periodic table of elements.
/GettyImages-186451122-58c399375f9b58af5cc2c1ac.jpg)
Occurrence
Physical Characteristics
- Sodium has an atomic number of 1 and atomic mass of 22.98. It is placed in group 1 of periodic table as it has a single electron in its outer most shell that it readily donates, creating a positively charged ion, the Na+ cation. At room temperature Sodium is soft, silvery-white metal which can be easily cut with a knife. It is highly reactive. In a...
Chemical Characteristics
- It is highly reactive in air and forms sodium hydroxide (NaOH), which is a strong base on reaction with water. In presence of air, sodium hydroxide film absorbs carbon dioxide, and lead to the formation of sodium bicarbonate. Sodium reacts with halogens under certain conditions to produce light. Halogen acids e.g. hydrofluoric and hydrochloric acid react vigorously with sodiu…
Significance and Uses
- Sodium is one of the most essential elements for life, as sodium and potassium keep a definite balance within the cell and are involved in maintaining an electrolyte balance across the cell membran...
- It helps in the regulation of blood pressure, blood volume, osmotic equilibrium and pH. Around 500mg of sodium is the minimum physiological requirement per day of human body.
- Sodium is one of the most essential elements for life, as sodium and potassium keep a definite balance within the cell and are involved in maintaining an electrolyte balance across the cell membran...
- It helps in the regulation of blood pressure, blood volume, osmotic equilibrium and pH. Around 500mg of sodium is the minimum physiological requirement per day of human body.
- Sodium Chloride is daily used as seasoning and preservative in diet. In plants, sodium is a micronutrient which helps in metabolism. It is involved in synthesis of chlorophyll, opening and closing...
- Significant amounts of sodium are used to produce synthetic detergents, dyes, intermediates of dye and perfumes.
Health Hazards
- According to The US Institute of Medicine, the Tolerable Upper Intake Level for sodium is 2.3 grams per day. A decrease in sodium intake would lead to fewer cases of hypertension . Hypertension causes almost 7.6 million premature deaths worldwide every year. The American Heart Association recommends not more than 1.5 g of sodium per day .
Isotopes of Sodium
- Twenty isotopes of sodium are identified, but only one, sodium-23 is stable. Two radioactive, cosmogenic isotopes which are the byproduct of cosmic ray spallation are known, Na-22 which has a half-life of 2.6 years and Na-24 with a half-life of 15 hours. All other isotopes have a half-life of less than one minute [10, 11].