
Metals In The Periodic Table
| Element | Symbol | Number In Periodic Table |
| Lithium | Li | 3 |
| Beryllium | Be | 4 |
| Sodium | Na | 11 |
| Magnesium | Mg | 12 |
Full Answer
What are the main metals in the periodic table?
The six alkaline earth metals are:
- beryllium
- magnesium
- calcium
- strontium
- barium
- radium
What are the most metallic elements in order?
- Oxygen is the most common element in the earth's crust
- Hydrogen is the most abundant element in the known universe, but only makes number 10 most abundant elements in the earth's crust
- Aluminum is the most abundant metal in the earth's crust.
What is the least metallic element in the periodic table?
What is the least metallic metal? Of solid and liquid elements at normal temperature and pressure, the least metallic are those at upper right of the Periodic Table: carbon, phosphorus, sulfur, bromine and iodine. Carbon in some of its allotropic forms has one property in common with metals; it is a good conductor of electricity.
What are the most important elements of the periodic table?
- Number Ten: Gallium. Gallium is a soft metal sometimes used in electronics.
- Number Nine: Copernicium.
- Number Eight: Curium.
- Number Seven: Francium.
- Number Six: Ununbium.
- Number Five: Bismuth.
- Number Four: Sulphur.
- Number Three: Helium.
What element is metal on the periodic table?
By definition, a metal element is an element that form positive ions and has metallic bonds. Most elements on the periodic table are metals. Examples of metal elements include iron, copper, silver, mercury, lead, aluminum, gold, platinum, zinc, nickel and tin.
What are the 20 elements of metal?
Metals in the first twenty elements are Lithium, Beryllium, Sodium, magnesium, Aluminum, Potassium, and calcium. Now the non-metals in the first twenty elements are Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon.
How many metals are on the periodic table?
There are about 70 metals out of 92 natural elements in the periodic table. How many metals are there among artificial elements created by mankind?
What are the 10 example of metal?
Examples of metals are aluminium, copper, iron, tin, gold, lead, silver, titanium, uranium, and zinc.
What are the 22 nonmetals?
In the above table nonmetal elements are H,He,C,N,O,F,Ne,P,S,Cl,Ar,Se,Br,Kr,I,Xe,At and Rn.
What are the first 20 metals?
Lithium, Beryllium, Sodium, Magnesium, Aluminium, Potassium, and Calcium are metals in the first twenty elements. Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon are the non-metals in the first twenty elements.
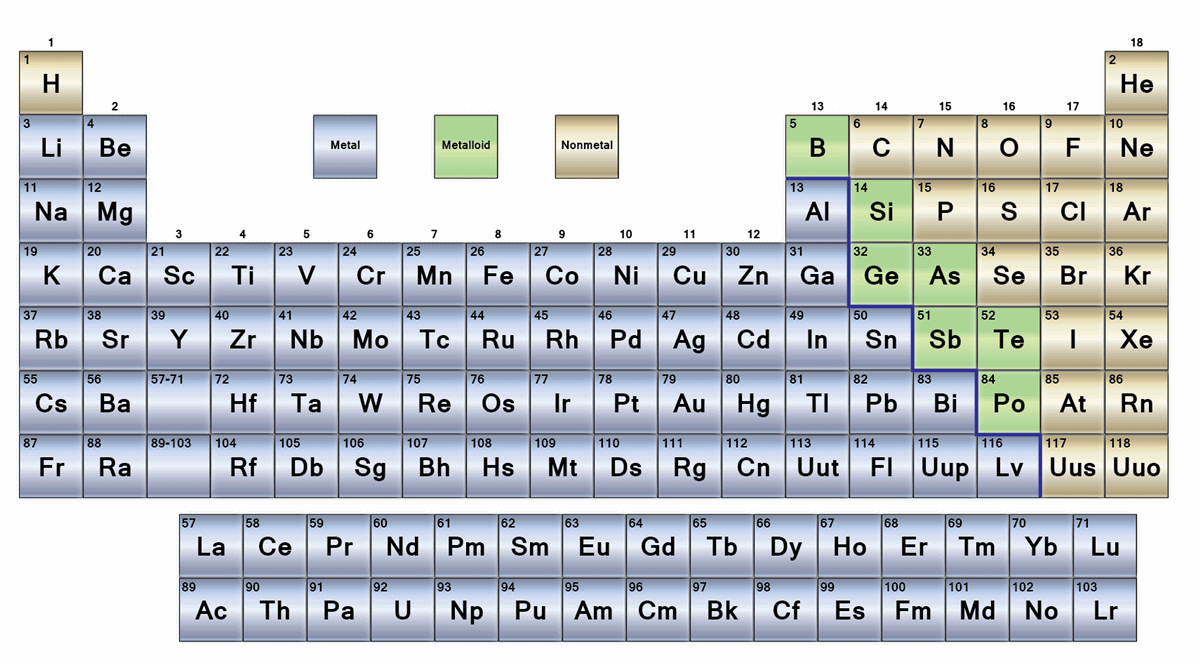
How do you tell if an element is a metal or nonmetal?
Elements to the left of the line are considered metals. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Elements to the far right of the periodic table are nonmetals. The exception is hydrogen (H), the first element on the periodic table.
Which of the following is a metal?
Answer: Sodium is a metal.
How many elements are metal and non-metal?
Out of the total 118 elements in the modern periodic table, 18 are non-metals, 7 are metalloids and 93 are metals.
What are the 17 non metals?
The 17 nonmetal elements are: hydrogen, helium, carbon, nitrogen, oxygen, fluorine, neon, phosphorus, sulfur, chlorine, argon, selenium, bromine, krypton, iodine, xenon, and radon.
Is Diamond a metal?
Diamond is not a metal in anyway its just an allotrope of carbon. It does not show any physical properties or chemical properties of metals like electrical conductivity, malleability, ductility, reaction with acids or salts etc.
What are the 5 metal elements?
Five MetalsIron.Uranium.Sodium.Aluminum.Calcium.
What is the name of 20 elements?
The Elements, sorted by Atomic NumberAtomic NumberSymbolName18ArArgon19KPotassium20CaCalcium21ScScandium76 more rows
Which of the first 20 elements are metalloids?
Metalloids : Boron and Silicon.
What are the first 20 elements of the periodic table?
The first 20 Elements of the Periodic Table: Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Sodium, Magnesium, Aluminium, Silicon, Phosphorus, Sulphur, Chlorine, Argon, Potassium, Calcium. The symbol for sodium is "Na".
How can I remember the first 20 elements?
0:155:18Trick to Learn First 20 Elements of the Periodic Table - YouTubeYouTubeStart of suggested clipEnd of suggested clipIt hi wrethic loves bbc news of neet nature. Here this h stands for hydrogen. This h stands for h eMoreIt hi wrethic loves bbc news of neet nature. Here this h stands for hydrogen. This h stands for h e helium. This l stands for l i lithium. This b stands for b e beryllium. This b stands for b boron.
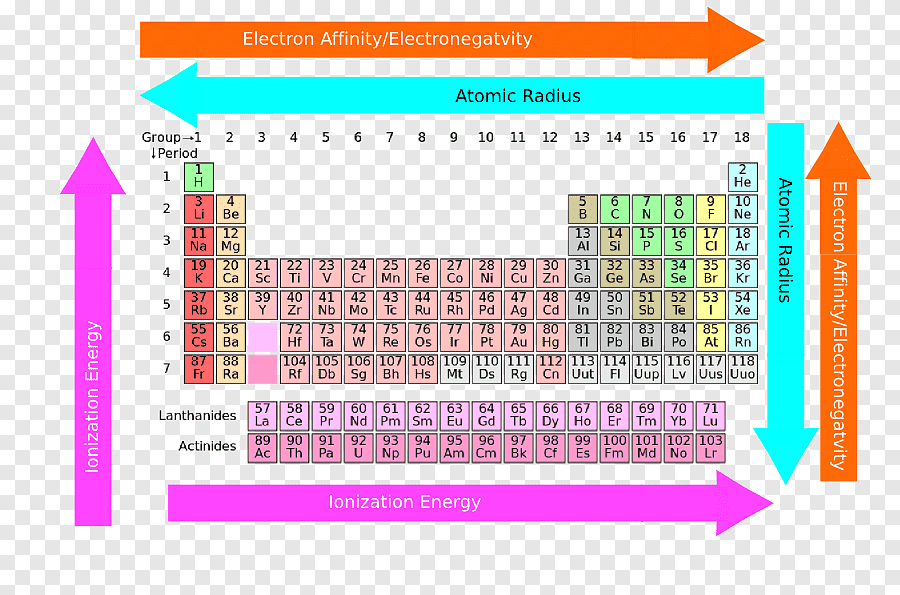
Where are the metals on the periodic table?
All of the metals are grouped together on the left side of the periodic table . Notice that hydrogen, colored red, is grouped with the metals in the top left corner. Even though it is grouped with the metals, hydrogen is considered to be a nonmetal.
What Are Metals?
Metals are elements that lose electrons easily, that are lustrous (reflective), malleable (can be molded into other shapes), and are good conductors of heat and electricity.
How do metals bond?
Metals bond by sharing electrons in what is known as metallic bonding. Metals are important to humans in a variety of ways. Metals can be used to make tools, build structures, and conduct electricity and heat. Metal ions play different vital roles in the body, and metals have antibiotic properties.
What are the elements on the periodic table that are malleable?
Metals are elements on the periodic table that are malleable, lose electrons easily, good conductors of heat or electricity, and typically appear reflective. Learn about the groupings of metals and nonmetals on the periodic table, features in metallic bonding and reactivity, and their formation of ionic compounds. Updated: 10/13/2021
What is the reactivity of metals?
Reactivity refers to the tendency of a metal to react with chemicals in its surroundings. The reactivity of metals varies greatly. Some metals, such those in columns 1 and 2 on the periodic table (potassium and sodium are examples), react easily with many different chemicals and are rarely found in their pure, elemental form. Both of these metals are usually only found in compounds (bonded to one or more other elements) or as ions (a charged version of their elemental form).
What is the bonding of metals?
Metallic Bonding. Many of a metal's remarkable and useful qualities stem from the way that metal atoms bond with each other, known as metallic bonding. Metallic bonding is how metal atoms interact on the atomic level; it is how metal atoms connect to make larger metal structures.
What is the reaction between pure metals and pure nonmetals?
When pure metals and pure nonmetals react, the nonmetals typically steal electrons from the metals in what is known as an oxidation-reduction, or redox, reaction. Rust is an example of a redox reaction. When oxygen from the atmosphere steals electrons from iron metal, rust, or iron oxide, forms. Lesson Summary.
What are metals in Periodic table?
Metals are the elements which have the tendency to donate or lose electrons to form positive ions.
Where are metals located on the periodic table?
The metals are located on the left side of the Periodic Table.
How many electrons do metals lose in a chemical reaction?
The atoms or metals have generally 1, 2 or 3 electrons in the outermost orbit, and they lose these electrons during a chemical reaction.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Which group of metals is the most reactive?
They are the Alkali metals of group 1. In 1st group, as we move down from top to bottom, the reactive of metals increases. Thus the bottom most element of group 1 (i.e francium) is the most reactive metal on the Periodic table. ( Note: Francium is a laboratory made element.
Why do metals make a ringing sound?
Metals produce ringing sound when they are stuck hard. This indicates that metals are sonorous in nature.
What are the elements in group 3 to group 12?
The elements lying in group 3 to group 12 are known as Transition metals (or transition elements). Transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14. Thus these metals are known as “Transition metals”.
How many electrons are in an alkaline metal?
All the alkaline earth metals have 2 electrons in their outermost orbit.
Where are transition metals found?
Transition metals are found in the middle part of the Periodic table (from Group 3 to group 11).
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
What is the inner transition metal?
The answer is: These elements have somewhat similar properties like that of transition metals, plus they are the elements of group 3 only, but they are placed at the bottom of the Periodic table as the inner section of group 3. Hence they are known as inner transition metals.
Where are alkaline earth metals located?
Alkaline earth metals are located on the left side of the Periodic table in group 2. Alkaline earth metals are also the reactive type of metals but they are less reactive as compared to alkali metals. All the alkaline earth metals have 2 electrons in their outermost orbit.
Which metal is the most reactive?
Alkali metals are the most reactive type of metals from the entire Periodic table of elements. As we move down the group from top to bottom in the group 1, the reactivity of alkali metals increases. List of alkali metals with atomic number, symbol and name. Atomic number. Symbol.
What color is heavy metal?
These heavy metals are displayed on the Periodic table with red color (see above image)
Where are metals located on the periodic table?
In general, metals are located on the left-hand side of the periodic table, decreasing in metallic character moving up and to the right. Depending on conditions, elements belonging to the metalloid group may behave like metals. In addition, even nonmetals may be metals.
Which element is a nonmetal?
For example, one allotrope of tin behaves more as a nonmetal. While most metals are hard, lead and gallium are examples of elements that are soft. These elements tend to have lower melting and boiling points than the transition metals (with some exceptions). Aluminum. Gallium.
What are the transition metals?
The transition metals are characterized by having partially filled d or f electron subshells. Since the shell is incompletely filled, these elements display multiple oxidation states and often produce colored complexes. Some transition metals occur in pure or native form, including gold, copper, and silver. The lanthanides and actinides are found only in compounds in nature.
What is the alkaline earth metal?
Alkaline Earth Metals. The alkaline earth metals are found in group IIA of the periodic table, which is the second column of elements. All of the alkaline earth metal atoms have a +2 oxidation state. Like the alkali metals, these elements are found in compounds rather than pure form. Alkaline earths are reactive but less so than alkali metals.
What are the characteristics of basic metals?
Basic Metals. The basic metals display the characteristics people generally associate with the term "metal.". They conduct heat and electricity, have a metallic luster, and tend to be dense, malleable, and ductile. However, some of these elements display nonmetallic characteristics.
Why are hydrogen and other elements highly reactive?
They are highly reactive elements, distinctive because of their +1 oxidation state and generally low density compared to other metals. Because they are so reactive, these elements are found in compounds. Only hydrogen is found free in nature as a pure element, and that is as diatomic hydrogen gas.
Is lanthanide a transition metal?
Although separate on the periodic table, lanthanides and actinides are really specific types of transition metals. Here's a list of all the elements on the periodic table that are metals.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
Which part of the periodic table is metal?
In other words, metals have a tendency to lose electrons. So the elements having metallic nature are found on the left and middle part of the Periodic table as shown in the above image.
How many elements are metallic?
More than 78% of the elements of the periodic table are metals. Out of the 118 known elements, there are around 94 elements which show metallic characteristics. But this number is not exact.
How many nonmetals are there in the periodic table?
How many Nonmetals are on the periodic table? There are total 18 Nonmetals on the Modern Periodic table. ( Note: Astatine shows some properties of nonmetals as well as some properties of metalloids. But it is classified as a nonmetal on the Periodic table.
What are elements that have metallic properties as well as nonmetallic properties called?
They are found between the metals and nonmetals. Elements which have the metallic properties as well as nonmetallic properties are classified as Metalloids. Because of this reason, they are also known as semimetals.
Which element is in the top right corner of the periodic table?
Elements which are in the top right corner of the Periodic table are classified as Nonmetals (Hydrogen is also a nonmetal which is located in the group 1). Nonmetals have the tendency to gain the electrons during a chemical reaction. In other words, the elements which gain electrons during a chemical reaction are classified as Nonmetals.
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
Is polonium a metalloid?
Astatine and Polonium also shows some characteristics of Metalloids.
Alkali Metals
Alkaline Earth Metals
- The alkaline earth metals are found in group IIA of the periodic table, which is the second column of elements. All of the alkaline earth metal atoms have a +2 oxidation state. Like the alkali metals, these elements are found in compounds rather than pure form. Alkaline earths are reactivebut less so than alkali metals. Group IIA metals are hard an...
Basic Metals
- The basic metalsdisplay the characteristics people generally associate with the term "metal." They conduct heat and electricity, have a metallic luster, and tend to be dense, malleable, and ductile. However, some of these elements display nonmetallic characteristics. For example, one allotrope of tin behaves more as a nonmetal. While most metals are hard, lead and gallium are examples …
Transition Metals
- The transition metalsare characterized by having partially filled d or f electron subshells. Since the shell is incompletely filled, these elements display multiple oxidation states and often produce colored complexes. Some transition metals occur in pure or native form, including gold, copper, and silver. The lanthanides and actinides are found only in compounds in nature. 1. Scandium 2…
More About Metals
- In general, metals are located on the left-hand side of the periodic table, decreasing in metallic charactermoving up and to the right. Depending on conditions, elements belonging to the metalloid group may behave like metals. In addition, even nonmetals may be metals. For example, in certain situations, you may find metallic oxygen or metallic carbon.