Who made the periodic table and why?
The periodic table was invented by chemist Dmitri Mendeleev to organize and compare elements and understand their relations with each other. Mendeleev created the periodic table between 1868 and 1870 while writing his book titled "The Principles of Chemistry." Initially, Mendeleev created the chart for his personal benefit, but others quickly discovered its value, leading to its immediate ...
Who developed Your modern day periodic table?
Mendeleev is the pioneer of the modern periodic table, after a numerous number of changes to the old version of the periodic table.
Who is the creator of the periodic table and why?
Why Was the Periodic Table Invented? The periodic table was invented by chemist Dmitri Mendeleev to organize and compare elements and understand their relations with each other. Mendeleev created the periodic table between 1868 and 1870 while writing his book titled “The Principles of Chemistry.”
Who was originally put the periodic table together?
The periodic table was originally put together by the Russian chemist Dmitri Mendeleev. He originally set up the periodic table around the existing 63 elements in 1869. He organized them in increasing atomic mass, and in periods (rows) and groups (columns) or specific properties.

Who created our modern periodic table?
chemist Dmitri MendeleevIn 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them.
Is Moseley the father of modern periodic table?
These elements' arrangement is according to a pattern. The periodic table was actually developed and by Mendeleev in the early 1800's. However, it was in fact perfected by Henry Moseley, an English physicist in 1913.
Why Mendeleev called father of the modern periodic table and not Meyer?
Answer and Explanation: Dmitri Mendeleev is considered the father of the modern periodic table because he was the first to create a table that classified elements by their atomic number. He created this table in 1869, and it has been used ever since.
Who is the father of all elements?
Dmitri MendeleevDmitri Mendeleev, Russian in full Dmitry Ivanovich Mendeleyev, (born January 27 (February 8, New Style), 1834, Tobolsk, Siberia, Russian Empire—died January 20 (February 2), 1907, St. Petersburg, Russia), Russian chemist who developed the periodic classification of the elements.
Who is Mendeleev and Moseley?
Mendeleev and Moseley are credited with being most responsible for the modern periodic law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties. The result is the periodic table as we know it today.
Who was Moseley Class 10?
Henry Moseley, in full Henry Gwyn Jeffreys Moseley, (born November 23, 1887, Weymouth, Dorset, England—died August 10, 1915, Gallipoli, Turkey), English physicist who experimentally demonstrated that the major properties of an element are determined by the atomic number, not by the atomic weight, and firmly established ...
What did Henry Moseley discover?
In 1913, while working at the University of Manchester, he observed and measured the X-ray spectra of various chemical elements using diffraction in crystals. Through this, he discovered a systematic relation between wave- length and atomic number. This discovery is now known as Moseley's Law.
What did Moseley do for the periodic table?
As a graduate student in Ernest Rutherford's physics laboratory at the University of Manchester in England, Moseley used newly discovered X-rays to redefine the Periodic Table, showing that it was actually organized by atomic number – the number of protons in an atom's nucleus – rather than by atomic weight, as ...
Who found the periodic table?
It has been the go-to reference on chemical elements for almost 150 years. Yet while the Russian chemist Dmitri Mendeleev is often credited with finding the rules behind the block-like patterns of elements, he was hardly alone: others had found them some years before, but failed to win recognition.
What did Mendeleev discover?
His confidence was vindicated with the discovery of gallium, germanium and scandium, ensuring his place among the great names of 19th-Century science.
Who discovered that elements with similar properties lie close together?
One of these scientists was John Newlands , an English chemist who in the mid-1860s pointed out that elements with similar properties lie close together if arranged according to their atomic mass.
Who created the table of elements?
Among the scientists who worked to created a table of the elements were, from left, Antoine Lavoisier, Johann Wolfang Döbereiner, John Newlands and Henry Moseley. In 1789, French chemist Antoine Lavoisier tried grouping the elements as metals and nonmetals.
What is the periodic table?
The periodic table of elements is a common sight in classrooms, campus hallways and libraries, but it is more than a tabular organization of pure substances . Scientists can use the table to analyze reactivity among elements, predict chemical reactions, understand trends in periodic properties among different elements and speculate on ...
What elements did Mendeleev predict?
The later discovery of elements predicted by Mendeleev, including gallium (1875), scandium (1879) and germanium (1886), verified his predictions and his periodic table won universal recognition. In 1955 the 101st element was named mendelevium in his honor. The 1869 periodic table by Mendeleev in Russian, with a title that translates "An experiment ...
Why is the periodic table important?
The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities between them. Scientists use the table to study chemicals and design experiments. It is used to develop chemicals used in the pharmaceutical and cosmetics industries and batteries used in technological devices.
What are the horizontal rows in the periodic table called?
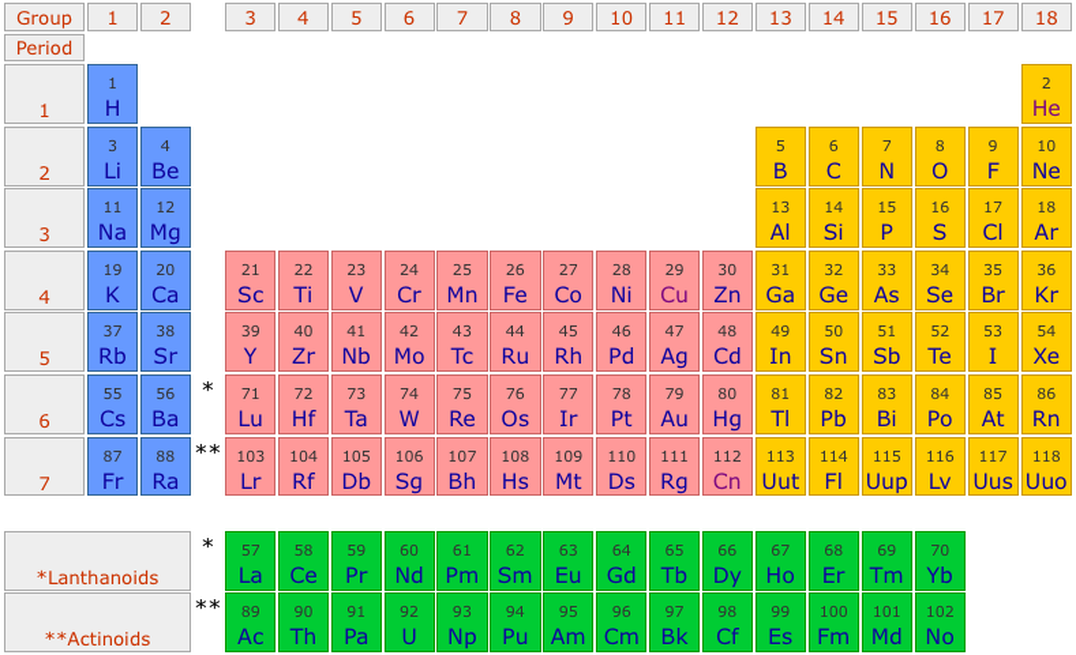
In the periodic table, the horizontal rows are called periods, with metals in the extreme left and nonmetals on the right. The vertical columns, called groups, consist of elements with similar chemical properties. The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities ...
What is the periodic table of chemical elements?
On its website marking the celebration, UNESCO wrote, “The Periodic Table of Chemical Elements is more than just a guide or catalogue of the entire known atoms in the universe; it is essentially a window on the universe, helping to expand our understanding of the world around us.”.
Why is the periodic table celebrated in 2019?
UNESCO named 2019 the International Year of the Periodic Table to mark the 150 th anniversary of Mendeleev’s publication. Researchers and teachers worldwide took this opportunity to reflect on the importance of the periodic table and spread awareness about it in classrooms and beyond.
Who invented the periodic table?
In the year 1869, a Russian chemist Dimitri Mendeleev started to develop the periodic table by arranging the chemical elements by atomic mass. He predicted the discovery of several other elements and left spaces open in his periodic table for them to accommodate. He is the one who invented the first periodic table and is the periodic table founder.
Who discovered the modern periodic table?
However, as the development of the periodic table was gradually done, the modern periodic table was discovered by Henry Moseley. Share this with your friends.
What is the periodic table?
The periodic table refers to an arrangement of the chemical elements that are organized on the basis of their atomic numbers, their electron configurations and their recurring chemical properties. Elements are presented in the periodic table in the order of the increasing atomic number. The standard form of the table contains a grid ...
What was the actual development of the periodic table?
The actual development in the periodic table happened after the development of Mendeleev’s periodic table. He introduced a law which stated that the properties of a given element are the periodic function of their atomic masses. He then arranged elements in periods or horizontal rows and groups or vertical columns in the increasing order according ...
What is the symbol of chemistry?
The periodic table is for many people the symbol of chemistry. It is a single image in a tabular form which contains all the known elements in the universe that are combined into an easily readable table. There are many different patterns present in the table as well. All of these elements seem to fit together and connect to each other to form a readable table and, in turn, the image of chemistry. The thought of elements first came around in 3000 B.C. The Greek philosopher Aristotle had an idea that everything on the Earth was made up of these elements. In the ancient times, elements like gold and silver were easily accessible, however, the elements which Aristotle chose were Earth, Water, Fire, and Air.
How are elements arranged in order?
According to him, the elements can be arranged in an ascending order according to their atomic weights. He also admitted that in this arrangement every eighth element in a row had the same properties to that of the first element of the same row, which depicts the octaves of music.
What elements did Aristotle choose?
In the ancient times, elements like gold and silver were easily accessible, however, the elements which Aristotle chose were Earth, Water, Fire, and Air.
When are cations formed?
B) Cations are formed when an atom loses electrons.
What is the smallest unit of an element?
D) An atom is the smallest identifiable unit of an element.
How many isotopes are there in a fictional element?
A fictional element has two isotopes, each making up 50% of the population. Isotope 1 has a mass of 80.0 amu, Isotope 2 has a mass of 85.0 amu. Calculate the atomic mass of the fictional element. A fictional element has two isotopes and an atomic mass of 87.08 amu.
What is the smallest unit of an element?
D) An atom is the smallest identifiable unit of an element.
How many protons are in a carbon atom?
1/12 the mass of a carbon atom containing six protons and six neutrons.
