
See more
Who actually invented the periodic table?
Dmitri MendeleevAlbert GhiorsoPeriodic table/Inventors
Who created the periodic table and why?
In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them.
What was the first periodic table?
Mendeleev's periodic table, published in 1869, was a vertical chart that organized 63 known elements by atomic weight. This arrangement placed elements with similar properties into horizontal rows.
Why is it called the periodic table?
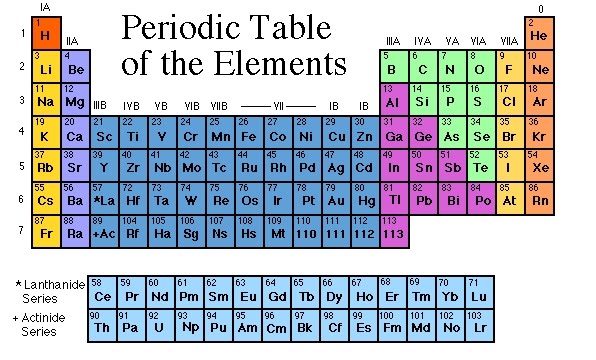
Why is the periodic table called the periodic table? It is called the periodic table because of the way the elements are arranged. You'll notice they're in rows and columns. The horizontal rows (which go from left to right) are called 'periods' and the vertical columns (going from up to down) are called 'groups'.
Why did Mendeleev create the periodic table?
He used them to correct properties of other elements such as the valence and also to predict properties of some three elements which were undiscovered by then; by putting them in order according to their atomic weights, he realized that physical and chemical properties were related to their atomic mass.
Why is the periodic table important?
The periodic table of elements puts all the known elements into groups with similar properties. This makes it an important tool for chemists, nanotechnologists and other scientists. If you get to understand the periodic table, and learn to use it, you'll be able to predict how chemicals will behave.
How and why is the periodic table arranged?
The chemical elements are arranged from left to right and top to bottom in order of increasing atomic number, or the number of protons in an atom's nucleus, which generally coincides with increasing atomic mass.
What did Henry Moseley contribute to the periodic table?
Moseley's contribution to the periodic table was that he arranged the elements in the periodic table according to atomic numbers. He realized his findings indicated that the identity of an element is how many protons it has. The number of protons represents the atomic number of an element.
Who invented the periodic table?
But it was the combined efforts of many chemists for the invention of Periodic table. The chemists who invented Periodic table are listed below. Antoine Lavoisier (1789) Johann Dobereiner (1829) Alexandre Beguyer de Chancourtois (1862) ...
When did the periodic table start?
I’ll tell you the complete History of Periodic table starting from 1789 to 1913.
Why Periodic table was invented?
The Periodic table was invented in order to classify all the known elements according to the similarities in their properties.
How did Johann Dobereiner classify the elements?
He classified the known elements by knowing their properties. After few years of this classification, several attempts were also made by other chemists. But some important work was given by Johann Dobereiner after few years. Let us see how Johann Dobereiner contributed to the development of Periodic table.
What are the unique identities of every element?
They also realized that the protons are the unique identity of every single element.
What did the king prepare for each known element?
He prepared the cards of each known element with their properties and details written on them.
How many elements are there in the universe?
Till today there are total 118 known elements like hydrogen, helium, lithium, beryllium, boron, carbon, and so on…
Who found the periodic table?
It has been the go-to reference on chemical elements for almost 150 years. Yet while the Russian chemist Dmitri Mendeleev is often credited with finding the rules behind the block-like patterns of elements, he was hardly alone: others had found them some years before, but failed to win recognition.
Who discovered that elements with similar properties lie close together?
One of these scientists was John Newlands , an English chemist who in the mid-1860s pointed out that elements with similar properties lie close together if arranged according to their atomic mass.
What did Mendeleev discover?
His confidence was vindicated with the discovery of gallium, germanium and scandium, ensuring his place among the great names of 19th-Century science.
When was the periodic table first published?
Periodic table at the Chemical Auditorium of the Gdańsk University of Technology, 1904. The first version of Mendeleev's periodic table, 1 March 1869 (N.S.): An attempt at a system of elements based on their atomic weights and chemical similarities.
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
How many categories are there in the periodic table?
The elements of the periodic table shown here are divided into nine categories; six for the metals, and two for nonmetals, and a metalloid category. The nine categories (or sets) correspond to those found in the literature for the applicable part of the periodic table. Different authors may use different categorisation schema depending on the properties of interest.
When is the 150th anniversary of the periodic table?
In celebration of the periodic table's 150th anniversary, the United Nations declared the year 2019 as the International Year of the Periodic Table, celebrating "one of the most significant achievements in science".
Who created the periodic table?
The periodic table was invented by Russian chemist Dmitri Mendeleev in 1869. However, prior to Mendeleev, chemists had been pondering for decades how to classify the elements. Beginning in 1789, Antoine Lavoisier began classifying elements by their properties. Johann Wolfgang Döbereiner showed in 1817 that elements could be arranged by their atomic ...
Who used atomic weights to classify the elements?
Atomic weights were used by English chemist John Newlands in 1864 in classifying the elements. After arranging the elements in order by atomic weight, Newlands noted that every eighth element seemed to have similar chemical properties. By analogy with the seven-note musical scale, he called this the law of octaves.
How is an element's position on the periodic table determined?
It was not until the early 20th century that it was discovered that an element’s position in the periodic table is determined by its atomic number (the amount of protons in its atomic nucleus).
How many columns did Mendeleev have in his 1869 table?
His 1869 table contained 17 columns (or groups, as they are now known). He revised this into an eight-group table in 1871. In his 1871 table, Mendeleev correctly predicted that the then known atomic weights of 17 elements were wrong.
What is the periodic table?
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, ...
Who proposed the periodic law?
Then in 1869, as a result of an extensive correlation of the properties and the atomic weights of the elements, with special attention to valency (that is, the number of single bonds the element can form), Mendeleyev proposed the periodic law, by which “the elements arranged according to the magnitude of atomic weights show a periodic change of properties.” Lothar Meyer had independently reached a similar conclusion, published after the appearance of Mendeleyev ’s paper.
What is the atomic number of an element?
The atomic number of an element is the number of protons in the nucleus of an atom of that element . Hydrogen has 1 proton, and oganesson has ...
What elements are triads?
Döbereiner in 1817 showed that the combining weight, meaning atomic weight, of strontium lies midway between those of calcium and barium, and some years later he showed that other such “ triads ” exist (chlorine, bromine, and iodine [halogens] and lithium, sodium, and potassium [alkali metals]). J.-B.-A. Dumas, L. Gmelin, E. Lenssen, Max von Pettenkofer, and J.P. Cooke expanded Döbereiner’s suggestions between 1827 and 1858 by showing that similar relationships extended further than the triads of elements, fluorine being added to the halogens and magnesium to the alkaline-earth metals, while oxygen, sulfur, selenium, and tellurium were classed as one family and nitrogen, phosphorus, arsenic, antimony, and bismuth as another family of elements.
Why do the elements in the periodic table have different orbits?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion principle, no more than two electrons can fill the same orbital. The first row of the periodic table consists of just two elements, hydrogen and helium. As atoms have more electrons, they have more orbits available to fill, and thus the rows contain more elements farther down in the table.
What are the elements that are related to the first seven?
Newlands proposed classifying the elements in the order of increasing atomic weights, the elements being assigned ordinal numbers from unity upward and divided into seven groups having properties closely related to the first seven of the elements then known: hydrogen, lithium, beryllium, boron, carbon, nitrogen, and oxygen . This relationship was termed the law of octaves, by analogy with the seven intervals of the musical scale.
Who proposed the atomic weights of the elements?
Attempts were later made to show that the atomic weights of the elements could be expressed by an arithmetic function, and in 1862 A.-E.-B. de Chancourtois proposed a classification of the elements based on the new values of atomic weights given by Stanislao Cannizzaro’s system of 1858.
Who created the table of elements?
Among the scientists who worked to created a table of the elements were, from left, Antoine Lavoisier, Johann Wolfang Döbereiner, John Newlands and Henry Moseley. In 1789, French chemist Antoine Lavoisier tried grouping the elements as metals and nonmetals.
What is the periodic table?
The periodic table of elements is a common sight in classrooms, campus hallways and libraries, but it is more than a tabular organization of pure substances . Scientists can use the table to analyze reactivity among elements, predict chemical reactions, understand trends in periodic properties among different elements and speculate on ...
What elements did Mendeleev predict?
The later discovery of elements predicted by Mendeleev, including gallium (1875), scandium (1879) and germanium (1886), verified his predictions and his periodic table won universal recognition. In 1955 the 101st element was named mendelevium in his honor. The 1869 periodic table by Mendeleev in Russian, with a title that translates "An experiment ...
Why is the periodic table important?
The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities between them. Scientists use the table to study chemicals and design experiments. It is used to develop chemicals used in the pharmaceutical and cosmetics industries and batteries used in technological devices.
What are the horizontal rows in the periodic table called?
In the periodic table, the horizontal rows are called periods, with metals in the extreme left and nonmetals on the right. The vertical columns, called groups, consist of elements with similar chemical properties. The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities ...
What is the periodic table of chemical elements?
On its website marking the celebration, UNESCO wrote, “The Periodic Table of Chemical Elements is more than just a guide or catalogue of the entire known atoms in the universe; it is essentially a window on the universe, helping to expand our understanding of the world around us.”.
Why is the periodic table celebrated in 2019?
UNESCO named 2019 the International Year of the Periodic Table to mark the 150 th anniversary of Mendeleev’s publication. Researchers and teachers worldwide took this opportunity to reflect on the importance of the periodic table and spread awareness about it in classrooms and beyond.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
What is PubChem working with?
PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Did Mendeleev's predictions get dismissed?
There were plenty of skeptics and it took years to gain international acceptance, but once newly-discovered elements matched the ones that Mendeleev predicted, his patterns could not be dismissed. In addition, some of the properties that he "fudged" were later recalculated and found to be much closer to his predictions.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
When was the periodic table updated?
The online version of the Periodic Table has been updated to the most recent printed version, which is dated September 2010. There was also a September 2009 version. Since version 4, elements have been added such that there are no "missing elements" from 1 to 118. Several official names have been added for high Z elements.
What is the atomic weight of ytterbium?
Redesign of the Periodic Table. Several numerical values have been updated. The standard atomic weight of ytterbium has been revised from 173.054 to 173.045. The values of the fundamental physical constants have been updated to the 2014 CODATA recommended values. For elements 103, 107, 108, 109, 111, 113, 115, the mass number of the longest-lived isotope has been updated.

Why Periodic Table Was invented?
Who Invented Periodic table?
- It was not the efforts of a single person (i.e Dimitri Mendeleev) behind the invention of Periodic table. It was the combined efforts of many scientists who contributed to the discovery of the Periodic table. Following chemists gave their valuable efforts in creating the Periodic table. 1. Antoine Lavoisier (1789) 2. Johann Dobereiner (1829) 3. Ale...
Final Arrangement
- After the valuable contribution of all the above mentioned scholars, many researchers worked on the atomic structure and finally the structure of atoms came into existence. Scientists realized that there is a heavy nucleus in the centre of atoms. This nucleus contains protons and neutrons in it. They also realized that the protons are the unique identity of every single element. The num…
Free Gift For You: Interactive Periodic Table
- Let me tell you how this Interactive Periodic Tablewill help you in your studies. 1).You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).You will get the detailed information about the periodic table which will convert a newbie into pro. 3).You will also get the HD images of the Periodic table (for FREE). Checkout Interactive Per…
The Inventor of The Periodic Table
Changes to The Table For Modern Science
- Mendeleev’s periodic table might have provided the foundation for the table we see hanging on the walls, but in the late 1800s, the basic structure of the atom had not been discovered yet. Chemists in the 1800s couldn’t view molecules, and things like protons and isotopes were unknown. They could organize elements by their atomic weight but not by their physical properti…
Reading The Periodic Table
- So, how do you read the periodic table? Each element is assigned a name, a symbol, an atomic weight and an atomic number. The elements are ordered by their atomic number, which indicates the number of protons inside the element’s nucleus and the number of electrons orbiting the nucleus. The number of protons and electrons is almost always equal. Hydrogen, for example, h…
The Importance of The Table
- Now that we know where the periodic table comes from, why is it so important for modern chemists? The reason is that it’s a shortcut to figuring out a bunch of information about each element. The element’s location on the table makes it easier for scientists to predict the kind of reactions that each component will have with the ones that surround it. Scientists organized the …
Summary
The periodic table, also known as the periodic table of the (chemical) elements, is a tabular display of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit a periodic dependence on their atomic numbers. Th…
Overview
The periodic table is a 2-dimensional structured table. The elements are placed in table cells, in reading order of ascending atomic number. The table columns are called groups, the rows are called periods. The breaks at the end of each period occur according to a repetition (or periodicity) of physical and chemical properties of the elements.
Periodic trends
As chemical reactions involve the valence electrons, elements with similar outer electron configurations may be expected to react similarly and form compounds with similar proportions of elements in them. Such elements are placed in the same group, and thus there tend to be clear similarities and trends in chemical behaviour as one proceeds down a group. As analogous configurations return …
Classification of elements
Many terms have been used in the literature to describe sets of elements that behave similarly. The group names alkali metal, alkaline earth metal, pnictogen, chalcogen, halogen, and noble gas are acknowledged by IUPAC; the other groups can be referred to by their number, or by their first element (e.g., group 6 is the chromium group). Some divide the p-block elements from groups 13 to …
History
In 1817, German physicist Johann Wolfgang Döbereiner began to formulate one of the earliest attempts to classify the elements. In 1829, he found that he could form some of the elements into groups of three, with the members of each group having related properties. He termed these groups triads. Chlorine, bromine, and iodine formed a triad; as did calcium, strontium, and barium; lithi…
Current questions
Although the modern periodic table is standard today, some variation can be found in period 1 and group 3. Discussion is ongoing about the placements of the relevant elements. The controversy has to do with conflicting understandings of whether chemical or electronic properties should primarily decide periodic table placement, and conflicting views of how the evidence should be used. A similar potential problem has been raised by theoretical investigations of the superheav…
Future extension beyond the seventh period
The most recently named elements – nihonium (113), moscovium (115), tennessine (117), and oganesson (118) – completed the seventh row of the periodic table. Future elements would have to begin an eighth row. These elements may be referred to either by their atomic numbers (e.g. "element 119"), or by the IUPAC systematic element names which directly relate to the atomic …
Alternative periodic tables
The periodic law may be represented in multiple ways, of which the standard periodic table is only one. Within 100 years of the appearance of Mendeleev's table in 1869, Edward G. Mazurs had collected an estimated 700 different published versions of the periodic table. Many forms retain the rectangular structure, including Janet's left-step periodic table (pictured below), and the m…