Dmitri Mendeleev
Dmitri Ivanovich Mendeleev was a Russian chemist and inventor. He formulated the Periodic Law, created a farsighted version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight eleme…
Why did Mendeleev structure gaps in the periodic table?
Mendeleev structured "gaps" into the periodic table in order to allow for as-yet-undiscovered elements which he predicted already existed (based on analysis and intuition) and would ultimately be discovered .
What did Mendeleev predict when he left empty spaces in his table?
Later when they were found, they fit in perfectly into the spaces Mendeleev predicted they would. He also predicted the properties of the unknown elements, and they were RIGHT.
What elements did Mendeleev predict?
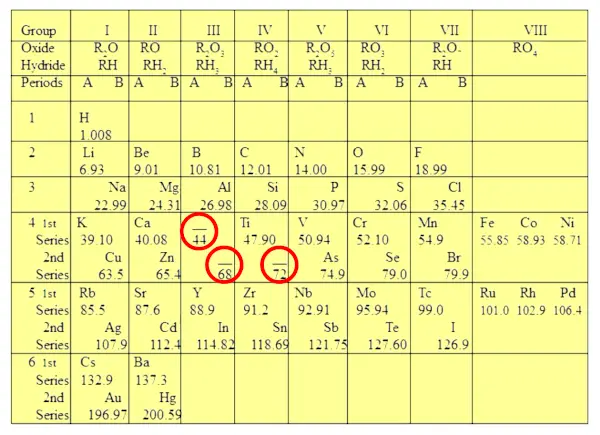
This left two gaps, which seemed to invalidate the working principle of the periodic system; but Mendeleev had the key intuition that those voids belonged to elements yet to be discovered - and he also predicted what their properties should be. It didn't take long for his vision to be vindicated: in about five years gallium and germanium were discovered and found to have the properties he had predicted; indeed, Mendeleev's predictions helped the discoverers telling them what they should look for.
What were the two attempts to make the periodic table?
Before Mendeleev made his version of the periodic table, other chemists designed theirs such that it was completely filled with the then known elements. Dobereiner triads and Newland's octaves were two attempts to make a successful periodic table. But they had their own demerits, like Newland's octave law only worked till the element Calcium. Dobereiner triads put different elements together and similar elements away in his table.
How did Mendeleev arrange his table?
Mendeleev arranged its table so that in each vertical column there were elements with similar properties. Sometimes the best fit was obtained jumping a slot: for example, when he arrived to arsenic - the first element heavier than zinc known at the time - he should have put it under aluminium, but these two elements have little in common; ditto for silicium. So he looked forward and found that arsenic in many ways resembles phosporus, and put id there.
Why did Mendeleev feel like he was constructing an ultimate jigsaw puzzle?
One can imagine Mendeleev feeling like he was constructing an ultimate jigsaw puzzle because organization of a cross of form and function of identification of elementalisms called for such allowance. Besides pseudoscience had exacted the table just by pretending a pretence of elementalism...much like having a beer right before there is permissiveness for doing so , like at the stroke of an hour, and you have a Stroh's...at strohour astrologic.
What was Mendeleev's organizing principle?
His organizing principle was to line up the elements by atomic mass and then arrange them in rows and columns so that their properties repeat in the columns so created. he did point out two obvious gaps in the data but missed a whopper, an entire column (the Inert Gases) of elements that do not show up on his table at all.
Answer
Mendeleev grouped elements according to similar physical properties, so he theorized that some elements had not yet been discovered. This is why he left gaps for those elements, along with possible descriptions and properties such as their melting points or appearances. These helped future chemists discover those elements.
New questions in Chemistry
the volume of a cylindrical tin can with a top and a bottom is to be 16Ï cubic inches. if a minimum amount of tin is to be used to construct the can, …
Answer
Mendeleev had left some gaps in his periodic table because he had believed that more elements would be discovered later.
New questions in Science
1. Which part of the plant carries out the process of photosynthesis? 2. Explain the role of guard cells in the process of plants making food ? 3. St …
Why did Mendeleev leave gaps in the periodic table?
Mendeleev left gaps in his periodic table because he knew that these elements existed, but had not yet been discovered. He believed that the elements would be eventually found and would fit perfectly into the gaps. Two such elements are Germanium and Gallium. 2.
What are the trends in atomic size, ionization energy, and electronegativity?
Describe the trends in atomic size, ionization energy, and electronegativity from left to right across a period in the periodic table. Within a period, the size of atoms decreases from left to right. The ionization energy increases when you move from left to right across a period.
What properties do metalloids display?
They are ductile and malleable. To the right of the stairstep, elements gain electrons when bonding.