What are facts about the periodic table?
Fun facts about the Periodic Table
- Carbon is unique in that it is known to form up to 10 million different compounds. ...
- Francium is the rarest element on earth. ...
- The only letter not in the periodic table is the letter J.
- The country Argentina is named after the element silver (symbol Ag) which is argentum in Latin.
What are the basic elements of the periodic table?
- Element 13 - Aluminum
- Element 31 - Gallium
- Element 49 - Indium
- Element 50 - Tin
- Element 81 - Thallium
- Element 82 - Lead
- Element 83 - Bismuth
- Element 113 - Ununtrium - will probably be a basic metal.
- Element 114 - Flerovium - will probably be a basic metal.
- Element 115 - Ununpentium - will probably be a basic metal.
What is the origin of the periodic table?
m The origins on the periodic table begin with the work of John Newlands and Dimitri Mendeleev. The origins of the periodic table lie in the work of a British scientist, John Newlands. In 1863, John Newlands divided the known 56 elements of matter into 11 groups. He did this based on their chemical properties and how they reacted.
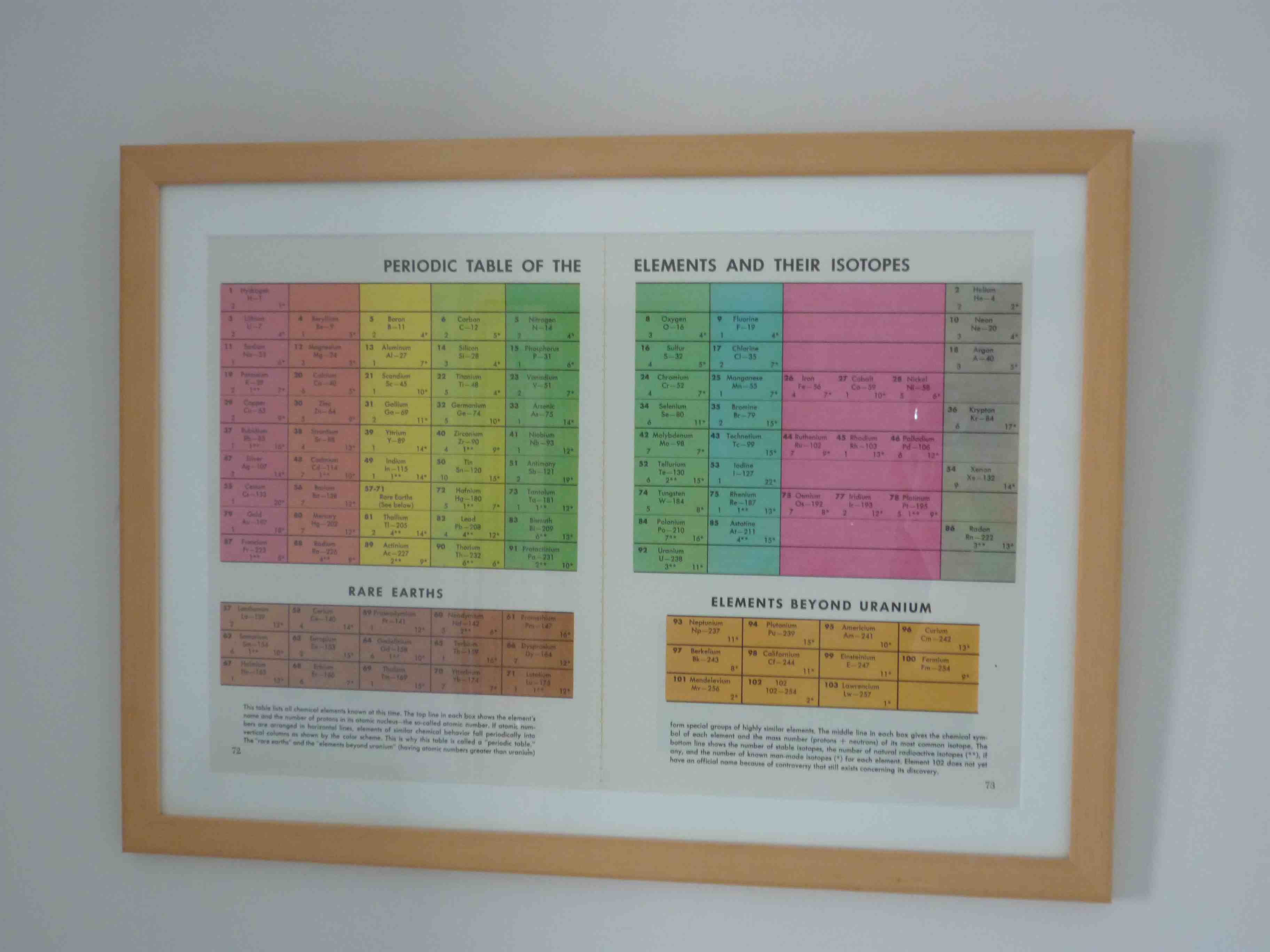
What do the numbers on the periodic table mean?
What do all the numbers mean on the periodic table? The number above the symbol is the atomic mass (or atomic weight). This is the total number of protons and neutrons in an atom. The number below the symbol is the atomic number and this reflects the number of protons in the nucleus of each element's atom.

Why is the periodic table organized?
Chart Organization. The organization of the periodic table allows you to predict the properties of the elements based on their position on the chart. Here's how it works: Elements are listed in numerical order by atomic number.
Why is the periodic table organized the way it is quizlet?
In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups.
How is the periodic table organized based on what?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
Why is the periodic table shaped weirdly?
By having the periodic table laid out in the shape of a castle, scientists can easily observe each element and its properties. If we were to order the chemical elements by alphabetical order, it would not be able to tell users anything about the properties of the elements.
How did Mendeleev organize the elements in the periodic table quizlet?
How did Mendeleev organize the elements in his periodic table? arranged the elements into rows in order of increasing mass so that elements with similar properties were in the same column.
Which statement best describes the organization of the periodic table of the elements?
Which statement best explains how periods on the Periodic Table are organized? Increasing atomic number from left to right.
Why is the periodic table organized by atomic number?
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).
Who created the periodic table and how it is organized?
In 1869 Russian chemist Dimitri Mendeleev started the development of the periodic table, arranging chemical elements by atomic mass. He predicted the discovery of other elements, and left spaces open in his periodic table for them.
How do you explain the periodic table to a child?
The periodic table is a system for arranging the chemical elements. The chemical elements are the basic substances that make up all matter. Each chemical element has a particular feature called its atomic number. That number comes from the amount of tiny particles called protons in each atom of the element.
Is there another way to organize the periodic table?
Charles Janet's left-step periodic table is the most widely used alternative to the traditional depiction of the periodic system. It organizes elements according to an idealized orbital filling (instead of valence).
What do all periodic tables have in common?
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties.
Why is it important that the periodic table is structured as a table rather than a list of elements?
The periodic table arranges the elements into families and periods (vertical and horizontal rows). The elements in each family have similar properties. As you go across a row, the properties vary gradually from one element to the next. The table tells you what elements may have similar chemical and physical properties.
What is the purpose of the periodic table quizlet?
What is the purpose of the periodic table? To organize various elements so that it is easy to study their atomic number, atomic mass, symbol, and mass.
Who created the periodic table and how is it organized quizlet?
Dmitri Mendeleev created the periodic table by arranging his elements in order of increasing atomic mass. He found out that when they were organized in this way they form a repeating pattern that periodically repeated every seven elements.
What are the groups of the periodic table organized by quizlet?
They are organized by the atomic number from least to greatest. They are also organized using groups similar properties. What information does the periodic table contain? The periodic table provides the atomic number, atomic mass, symbol and name.
How did Mendeleev originally organize the periodic table?
Mendeleev arranged the elements in order of increasing weight and broke them into rows such that elements in each column shared valence, the number of other atoms they combined with, as well as other properties.
How does the periodic table work?
Here's how it works: Elements are listed in numerical order by atomic number. The atomic number is the number of protons in an atom of that element. So element number 1 (hydrogen) is the first element.
Why is the periodic table important?
The periodic table is one of the most valuable tools for chemists and other scientists because it orders the chemical elements in a useful way. Once you understand how the modern periodic table is organized, you'll be able to do much more than just look up element facts like their atomic numbers and symbols.
What are the trends in the periodic table?
As you progress in chemistry, there are other trends in the periodic table you'll need to know: 1 Atomic radius and ionic radius increase as you move down a group, but decrease as you move across a period. 2 Electron affinity decreases as you move down a group, but increases as you move across a period until you get to the last column. The elements in this group, the noble gases, have practically no electron affinity. 3 The related property, electronegativity, decreases going down a group and increases across a period. Noble gases have practically zero electronegativity and electron affinity because they have complete outer electron shells. 4 Ionization energy decreases as you move down a group, but increases moving across a period. 5 Elements with the highest metallic character are located on the lower left side of the periodic table. Elements with the least metallic character (most nonmetallic) are on the upper right side of the table.
How many periods are there in the periodic table?
There are seven periods on the periodic table. Elements in the same period all have the same electron ground state energy level. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties.
What are the two main types of elements?
The two main types of elements are metals and nonmetals . There are also elements with properties intermediate between metals and nonmetals. These elements are called metalloids or semimetals. Examples of groups of elements that are metals include alkali metals, alkaline earths, basic metals, and transition metals.
What is the element symbol?
The element symbol is a shorthand notation that is either one capital letter or a capital letter and a lowercase letter. The exception is the elements at the very end of the periodic table, which have placeholder names (until they are officially discovered and named) and three-letter symbols.
What is the atomic number of an element?
Every atom of hydrogen has 1 proton. Until a new element is discovered, the last element on the table is element number 118 . Every atom of element 118 has 118 protons.
How are the columns in the periodic table organized?
The rows and columns are organized by precise characteristics. The elements that are in the same column or in the same rows have common characteristics. For example, magnesium (Mg) and sodium ...
What is the periodic table called?
Let us investigate periods. After all, that is how the periodic table gets its name. Each of the rows from left to right is called a period. What that means in that each and every one of the elements in a row shares similar electron configurations with the others. Or, in other words, each of the elements in the same row has the exact same number of atomic orbitals.
Why are valence electrons important?
— Marty Rubin. All the elements in each group have the same number of electrons in their outer orbitals, also known as valence electrons. These electrons are important because they are involved in the chemical bonds with other elements.
How many orbitals does an element have?
If you look at all the elements on the top row or, in other words, the elements in the first period, you will see that all of them have one atomic orbital for their electrons. Then, the elements on the second row, or second period, are characterized by having two atomic orbitals in their electrons.
How to read valence electrons?
You have to read groups from left to right. All the elements in the first column, or group one, have one valence electrons (one electron in their outer shell). All the elements in the second column, or group two, have two valence electrons. But all the elements in the third group (group three), have thirteen valance electrons. From then on, you have to add an electron for every group until reaching 18. Simply, counting the columns will allow you to know how many electrons each element has on its outer shell. There are a few exceptions to this, though, because some elements are transition elements that add electrons.
Why do all elements have one thing in common?
Because they all have one thing in common: their respective valence shells are full. This is how the periodic table is organized. Understanding that the position of each and every one of the elements is useful in understanding their properties.
How many valance electrons are in the third group?
But all the elements in the third group (group three), have thirteen valance electrons. From then on, you have to add an electron for every group until reaching 18. Simply, counting the columns will allow you to know how many electrons each element has on its outer shell.
Why is the periodic table called the periodic table?
The periodic table's name comes from the fact that it arranges the elements into repeating sets, otherwise known as "periods.". These periods are defined by the covalence of an element, the number of electrons it has in its outermost shell and by other elemental attributes. This arrangement places elements with similar chemical properties close ...
Who was the first scientist to discover the attributes of certain groups of chemical elements?
Russian chemist and inventor Dmitri Mendeleev was one of the first scientists to realize the attributes that certain groups of chemical elements had in common. He used this observation and formed one of the earliest versions of the periodic table.
Did Mendeleev's table include all elements?
Mendeleev's table did not include all of the elements that it does now, as many elements had yet to be discovered at that time. However, his table was able to predict that newly discovered elements would fit certain profiles and he designed the table to accommodate this.
