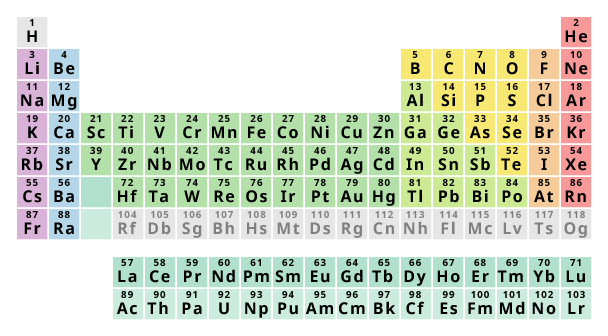
Why is Mendeleev given credit for the periodic table?
Why is Mendeleev generally given credit for the periodic table? Mendeleev is given more credit because he published his organizational scheme and went on to demonstrate its usefulness. Mendeleev predicted the existence of properties of undiscovered elements and left blanks in his periodic table for them.
Why is the periodic table organized the way it is?
Why was the periodic table organized the way it is? The arrangement of the periodic table was formulated in order to give a very informative representation of the chemical elements. Each of these elements is specifically placed in the periodic table, keeping specific parameters in mind.
Why is the periodic table divided into groups?
The Periodic table is divided into groups for the following reasons;. To classify the elements according to their similar properties. By dividing the elements in groups, it becomes very easy to study them. By dividing the Periodic table in groups, you can easily make a group wise comparison of elements.
What are facts about the periodic table?
Fun facts about the Periodic Table
- Carbon is unique in that it is known to form up to 10 million different compounds. ...
- Francium is the rarest element on earth. ...
- The only letter not in the periodic table is the letter J.
- The country Argentina is named after the element silver (symbol Ag) which is argentum in Latin.

Why is it called the periodic table and not just the table of elements?
The periodic table's name comes from the periodic behavior of its constituent components. Periodicity develops as a result of the repeating of elements with identical outer electronic configurations at a regular interval of time.
Why is the table of elements called the periodic table quizlet?
Why is the table in the elements shown in figure 7 called a periodic table? because the properties of the elements repeat at regular intervals from row to row.
What is called periodic table?
What is the periodic table? The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson.
Who created the periodic table?
Dmitri MendeleevAlbert GhiorsoPeriodic table/Inventors
How are elements arranged in the periodic table?
The chemical elements are arranged in order of increasing atomic number. The horizontal rows are called periods and the vertical columns are called groups. Elements in the same group have similar chemical properties. This is because they have the same number of outer electrons and the same valency.
How do you explain periodic table to a child?
The periodic table is a system for arranging the chemical elements. The chemical elements are the basic substances that make up all matter. Each chemical element has a particular feature called its atomic number. That number comes from the amount of tiny particles called protons in each atom of the element.
Why is the periodic table arranged that way?
Mendeleev arranged the elements in order of increasing weight and broke them into rows such that elements in each column shared valence, the number of other atoms they combined with, as well as other properties.
What is the periodic table of the elements quizlet?
a table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure (and hence similar chemical properties) appear in vertical columns.
Why are there only two elements in the first period of the periodic table quizlet?
Solution. The p orbital does not exist for energy level 1. The first energy level consists only of a single s orbital that holds a maximum of two electrons.
What are the rows of the periodic table called quizlet?
The rows in the periodic table are called periods, and each of the elements in a row has the same number of energy levels. The columns in the table are called groups, and all of the elements in a column are structurally similar and form similar bonds with other elements.
What is a row of elements on the periodic table called?
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column.
Why is the periodic table called the periodic table?
Periodic table is called so because of the arrangement of elements in it. The elements are arranged in such a way that their properties repeat after regular intervals. You know that the Helium (He) is the 2nd element on the Periodic table.
What is periodic table?
Definition: Periodic table of elements is the row wise and column wise arrangement of chemical elements in the increasing order of their atomic number. This arrangement of elements is done in such a way that the elements lying in the same column have similar properties. This is the short and simple definition of Periodic table.
What is the 8th element after helium?
Now if we count the 8th element after helium, then that element is Neon (Ne). This element has atomic number 10 and it is also an inert gas. Again if we count the 8th element after Neon (Ne), then that element is Argon (Ar). This element has atomic number 18 and it is also an inert gas.
What element is after Argon?
Again if we count the 18th element after Argon (Ar), then that element is Krypton (Kr).
Which group of periodic table has the same number of valence electrons?
Again, the group 2 of Periodic table are Alkaline earth metals and they also have similar properties. Similarly each group on the Periodic table have the elements which show similar physical as well as chemical properties. Also the elements lying in the same group have the same number of valence electrons.
What does table mean in math?
Table: Table indicates the arrangement in rows and columns
Which column of the periodic table has the most reactive metals?
For example, first column of Periodic table (also known as group 1) have the metals which are highly reactive. They are known as alkali metals. All these metals of first group have similar properties. Again, the group 2 of Periodic table are Alkaline earth metals and they also have similar properties. Similarly each group on the Periodic table have ...
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).