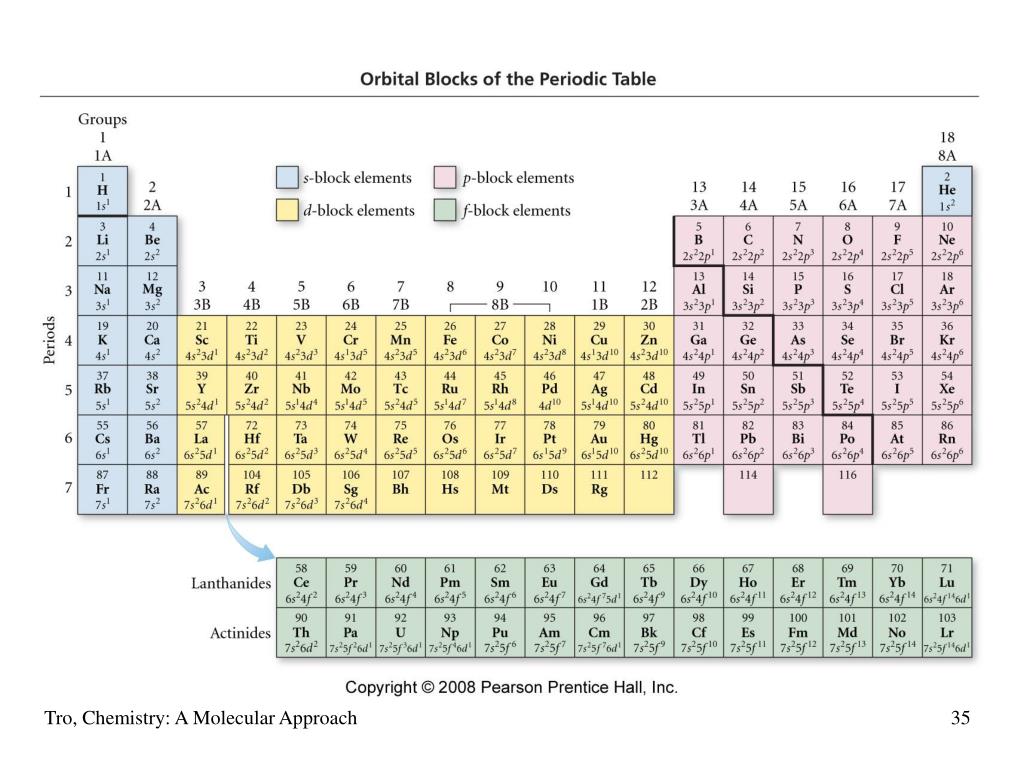
Which element has 3 energy levels and 5 valence electrons?
The element that has three energy levels and five valence electrons in its outer level is phophorous, atomic number 15. What element has 5 valence electrons and 6 energy levels? What element has 5 electron shells and 6 valence electrons? Does PB have 5 valence electrons?
How many valence electrons does phosphorus have in the periodic table?
Group 1 elements have one valence election, Group 2 elements have two, Group 13 (also known as IIIA) have three, Group 14 (IVA) have four, Group 15 (VA) have five and so on. Phosphorous is the 5th element from the left in the third period so it has five valence electrons.
How many electron energy levels are there in the 3rd period?
All elements in the third period have three electron energy levels and have their valence electrons in the third level. Orbitals are sub-levels within the principal energy level, so the word orbital isn't used interchangeably with energy level.
What is the principal energy level of the periodic table?
Each row or period of the periodic table corresponds to a principal energy level. All elements in the third period have three electron energy levels and have their valence electrons in the third level. Orbitals are sub-levels within the principal energy level, so the word orbital isn't used interchangeably with energy level.
Which element has three energy levels and five valence electrons in its outer level?
How many valence electrons does phosphorus have?
Which element has three energy levels?
About this website

What element has 5 valence electrons and 3 shells?
A neutral phosphorus atom has 15 total electrons. Two electrons can go into first shell, eight in the second shell, and it has five more in the third shell. The third shell is the outer valence shell, so it has 5 valence electrons.
What elements has 5 valence electron?
Elements having 5 valence electrons are placed in Group 15. The elements of group 15 are nitrogen, phosphorus, arsenic and antimony.
What element is in Group 5 period 3?
Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi)....Group 5A — The Pnictogens.3AB4AC5AN6AO7AF12 more columns
What period has 5 valence electrons?
fourth periodTherefore, the element in the fourth period of the periodic table which has five valence electrons is Arsenic.
Which element has 5 valence electrons for bonding?
nitrogenAtoms in the nitrogen family have 5 valence electrons. They tend to share electrons when they bond. Other elements in this family are phosphorus, arsenic, antimony, and bismuth.
Does boron have 5 valence electrons?
Boron is a chemical element with the chemical symbol B. Boron's atomic number is 5. Boron has an electron valence of 3, meaning it has 3 electrons within its valence shell. Its atomic configuration is1s22s22p1.
What is the Group 3 element in period 3?
Group 3A (or IIIA) of the periodic table includes the metalloid boron (B), as well as the metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). Boron forms mostly covalent bonds, while the other elements in Group 3A form mostly ionic bonds....Group 3A.31ANa2AMg4ASi12 more columns
Which among the following elements belong to period 3 in the periodic table?
The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon.
What is the name of Group 3 in periodic table?
scandium familyGroup namesNew IUPAC nameOld IUPAC (Europe)Name by elementGroup 3IIIAscandium familyGroup 4IVAtitanium familyGroup 5VAvanadium familyGroup 6VIAchromium family14 more rows
What element in period 3 has 4 valence electrons?
The number of valence electronsPeriodic table groupValence ElectronsGroup 13 (III) (boron group)3Group 14 (IV) (carbon group)4Group 15 (V) (pnictogens)5Group 16 (VI) (chalcogens)65 more rows•Jun 5, 2019
What is period 5 on the periodic table?
The period 5 transition metals are yttrium (Y), zirconium (Zr), niobium (Nb), molybdenum (Mo), technetium (Tc), ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), and cadmium (Cd).
Which element has more than 5 valence electrons?
Valence Electron of ElementsPeriodic Table GroupValence ElectronsNitrogen group – Group 15 (V)5Oxygen group – Group 16 (VI)6Halogens – Group 17 (VII)7Noble gases – Group 18 (VIII or 0)84 more rows•May 8, 2020
Which element has more than 5 valence electrons?
Valence Electron of ElementsPeriodic Table GroupValence ElectronsNitrogen group – Group 15 (V)5Oxygen group – Group 16 (VI)6Halogens – Group 17 (VII)7Noble gases – Group 18 (VIII or 0)84 more rows•May 8, 2020
What is the valency of group 5 elements?
Group 5 elements, however, have 5 valence electrons and will tend to take 3 electrons and so have a valency of -3.
How many covalent bonds are formed with 5 valence electrons?
three covalent bondsGroup 5A (15) elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia).
What is the valence electron of elements?
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell.
Which element has three energy levels and five valence electrons in its outer level?
The element that has three energy levels and five valence electrons in its outer level is phophorous, atomic number 15. Each row or period of the periodic table corresponds to a principal energy level. All elements in the third period have three electron energy levels and have their valence electrons...
How many valence electrons does phosphorus have?
Phosphorous is the 5th element from the left in the third period so it has five valence electrons.
Which element has three energy levels?
The element that has three energy levels and five valence electrons in its outer level is phophorous, atomic number 15. Each row or period of the periodic table corresponds to a principal energy level. All elements in the third period have three electron energy levels and have their valence electrons in the third level. Orbitals are sub-levels within the principal energy level, so the word orbital isn't used interchangeably with energy level.
Which element has three energy levels and five valence electrons in its outer level?
The element that has three energy levels and five valence electrons in its outer level is phophorous, atomic number 15. Each row or period of the periodic table corresponds to a principal energy level. All elements in the third period have three electron energy levels and have their valence electrons...
How many valence electrons does phosphorus have?
Phosphorous is the 5th element from the left in the third period so it has five valence electrons.
Which element has three energy levels?
The element that has three energy levels and five valence electrons in its outer level is phophorous, atomic number 15. Each row or period of the periodic table corresponds to a principal energy level. All elements in the third period have three electron energy levels and have their valence electrons in the third level. Orbitals are sub-levels within the principal energy level, so the word orbital isn't used interchangeably with energy level.
.PNG)