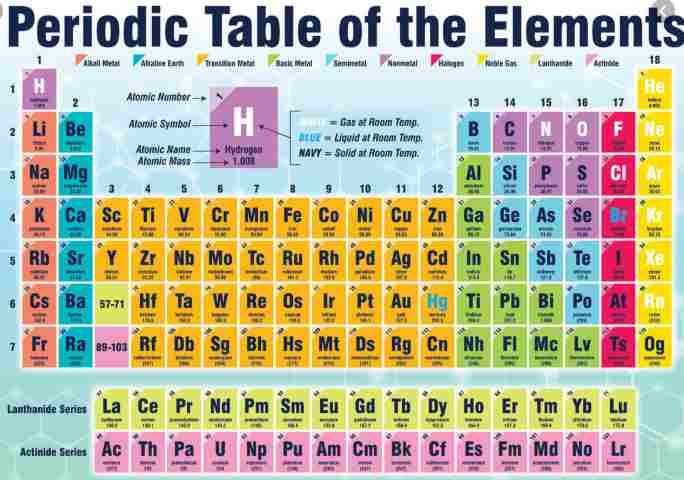
What is Group 4 on the periodic table?
Group 4 is a group of elements in the periodic table. It contains the elements titanium (Ti), zirconium (Zr), hafnium (Hf) and rutherfordium (Rf). This group lies in the d-block of the periodic table. The group itself has not acquired a trivial name; it belongs to the broader grouping of the transition metals.
What is a group or family on the periodic table?
What is a family on the periodic table?
- Team 1: antacids steels, or lithium family.
- Team 2: alkaline planet steels, or beryllium family.
- Team 3: the scandium family.
- Team 4: the titanium family.
- Team 5: the vanadium family.
- Team 6: the chromium family.
- Team 7: the manganese family.
- Team 8: the iron family.
What are the groups and periods of the periodic table?
- Lithium (Li)
- Sodium (Na)
- Potassium (K)
- Rubidium (Rb)
- Cesium (Cs)
- Francium (Fr)
What are groups and columns in the periodic table called?
- The horizontal rows are called periods.
- The vertical columns are called groups.
- Elements in the same group are similar to each other.
- The metals are on the left and the non-metals are on the right (hydrogen is a non-metal but is often put in the middle).

Are there 8 or 18 groups in the periodic table?
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered.
How many groups are in the periodic table?
Groups are numbered from 1 to 18. From left to right in the periodic table, there are two groups (1 and 2) of elements in the s-block, or hydrogen block, of the periodic table; ten groups (3 through 12) in the d-block, or transition block; and six groups (13 through 18) in the p-block, or main block.
What are the 7 groups of the periodic table?
Different groups are present in the periodic table:The Alkali Metals.The Alkaline Earth Metals.The Transition Metals.The Non-metals.The Halogens.The Noble Gases.The Rare Earth Elements.
What are the 8 groups of the periodic table?
Name of the eight groups in the periodic table:Alkali metals.Alkaline earth metals.Rare earth metals.Crystallogens.Pnictogens.Chalcogens.Halogens.Noble gases.
How many elements are there in the periodic table of 2022?
Today, with 118 known elements, it is widely regarded as one of the most significant achievements in science.
What are the 18 groups of the periodic table?
Groups are numbered 1–18 from left to right. The elements in group 1 are known as the alkali metals; those in group 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in 18 are the noble gases.
Why are Group 7 called halogens?
Chlorine, bromine and iodine are the three common Group 7 elements. Group 7 elements form salts when they react with metals. The term 'halogen' means 'salt former'.
What are the 11 groups on the periodic table?
Group 11, by modern IUPAC numbering, is a group of chemical elements in the periodic table, consisting of copper, silver, and gold. Roentgenium is also placed in this group in the periodic table, although no chemical experiments have yet been carried out to confirm that it behaves like the heavier homologue to gold.
Why are there 18 groups in the periodic table?
The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity.
What is group 8 called?
Group 8A (or VIIIA) of the periodic table are the noble gases or inert gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
What is Group 13 called?
boron group element, any of the six chemical elements constituting Group 13 (IIIa) of the periodic table. The elements are boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh).
What are the 11 noble gases?
The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og). Noble gases are colourless, odourless, and tasteless nonflammable gases.
Why are there 18 groups in the periodic table?
The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity.
What are the 5 main groups of the periodic table?
5 Element FamiliesAlkali metals.Alkaline earth metals.Transition metals.Halogens.Noble gases.
What are the 5 types of elements?
According to some traditions, everything in the universe comes from the five elements: wood, fire, earth, water, and metal. From the smallest atom to a giant whale to the solar system itself, all things are said to be composed of some combination of these elements.
What are the 4 types of elements?
The Four Elements. Greek philosophy supposed the Universe to comprise four elements: Fire, Water, Earth, and Air.
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
What are the elements that chemists classify?
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
What are the different types of nonmetals?
The nonmetals, halogens, and noble gases are all types of nonmetals. The metalloids have properties intermediate between metals and nonmetals. The alkali metals, alkaline earths, lanthanides, actinides, transition metals, and basic metals are all groups of metals.
