What are the three general groups on the periodic table?
Periodic Table Groups. Group 1: Alkali Metals. Alkali metals are soft, ductile, and good conductors of electricity and heat. This group includes the elements Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium. Alkali metals are very reactive. Compared to other elements they have a low melting and boiling point. Group 2: Alkaline Earth ...
What are the groups and families on the periodic table?
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods. Group (family): A vertical column in the periodic table.
What is Group 10 on the periodic table called?
What is Group 10 on the periodic table called? Group 10, numbered by current IUPAC style, is the group of chemical elements in the periodic table that consists of nickel (Ni), palladium (Pd), platinum (Pt), and perhaps also the chemically uncharacterized darmstadtium (Ds). All are d-block transition metals.
How are groups on a periodic table numbered?
the main groups are numbered from 1 to 7 going from left to right, and the last group on the right is Group 0; ... Only elements are found in the periodic table, never compounds. For example ...
Are there 8 or 18 groups in the periodic table?
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered.
Are there 7 or 8 groups in the periodic table?
There is eighteen groups on the periodic table in total, and each periodic table group contains elements with the same number of valence electrons.
Why are there 8 groups in the periodic table?
The idea behind the periodic system is to find regularities (hence the name) between elements. It turns out, that individual groups and trends within them are similar in the 8 "main" groups (as you have learned).
What are the 8 groups of the periodic table?
Name of the eight groups in the periodic table:Alkali metals.Alkaline earth metals.Rare earth metals.Crystallogens.Pnictogens.Chalcogens.Halogens.Noble gases.
Are there 7 or 18 groups in the periodic table?
The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity.
What is Group 13 called?
boron group elementboron group element, any of the six chemical elements constituting Group 13 (IIIa) of the periodic table. The elements are boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh).
How many elements are there in the periodic table of 2022?
118Today, with 118 known elements, it is widely regarded as one of the most significant achievements in science.
What are the 7 periods in periodic table?
Period 7 elementHydrogenHeliumLithiumBerylliumNeonSodiumMagnesiumArgonPotassiumCalciumKryptonRubidiumStrontiumXenon2 more rows
What are the 18 groups of the periodic table?
There are total 18 different groups in Periodic table.Group 1: Alkali metals group (hydrogen not included)Group 2: Alkaline earth metals group.Group 3-12: Transition and Inner transition metals group.Group 13: Boron group.Group 14: Carbon group.Group 15: Nitrogen group.Group 16: Oxygen group.Group 17: Halogen group.More items...•
What are called noble gases?
noble gas, any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).
What are the 4 types of elements?
The four elements of western culture are: EARTH, AIR, FIRE, and WATER. These four elements were believed to be essential to life. Taoism has five elements, each one superior to the next in turn: wood, earth, water, fire, and metal.
What are group 5 elements called?
Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi).
What are the 7 groups of the periodic table?
Different groups are present in the periodic table:The Alkali Metals.The Alkaline Earth Metals.The Transition Metals.The Non-metals.The Halogens.The Noble Gases.The Rare Earth Elements.
What are the name of the 7 groups of a periodic table?
2. The terms used to describe groups and periods of the periodic table; alkali metals, alkali earth metals, halogens, noble gases, lanthanides and actinides, 3.
What are the 18 groups of the periodic table?
There are total 18 different groups in Periodic table.Group 1: Alkali metals group (hydrogen not included)Group 2: Alkaline earth metals group.Group 3-12: Transition and Inner transition metals group.Group 13: Boron group.Group 14: Carbon group.Group 15: Nitrogen group.Group 16: Oxygen group.Group 17: Halogen group.More items...•
What are the 7 periods in periodic table?
Period 7 elementHydrogenHeliumLithiumBerylliumNeonSodiumMagnesiumArgonPotassiumCalciumKryptonRubidiumStrontiumXenon2 more rows
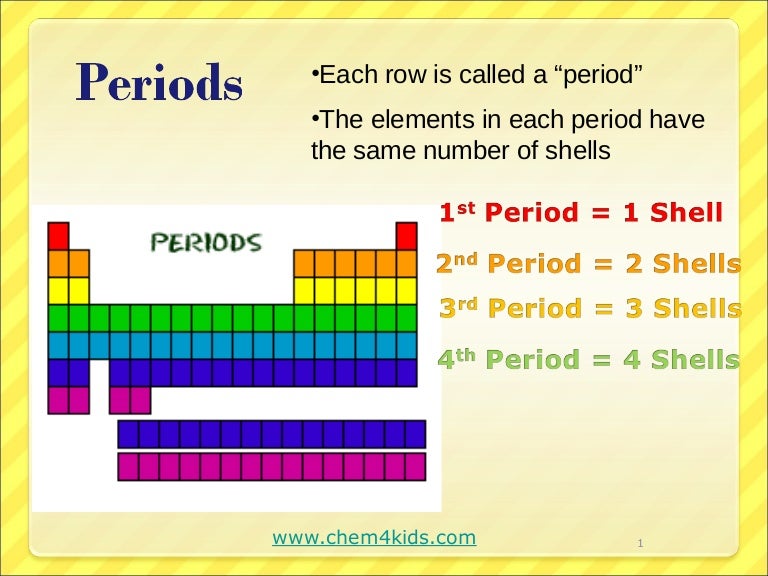
How many periods are there in the periodic table?
Elements within a period display periodic table trends, moving from left to right, involving atomic and ionic radius, electronegativity, There are seven element periods. Some periods contain more elements than others because the number of included elements depends on the number of electrons allowed in an energy sublevel.
What is the difference between periodic table groups and periods?
Periodic Table Groups and Periods. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Both groups and periods reflect the organization ...
What are the elements that chemists classify?
These groups go by the names alkali metals, alkaline earth metals, transition metals, basic metals, nonmetals, halogens, noble gases, lanthanides, and actinides.
How many valence electrons are in group 17?
For example, elements in group 1 have 1 valence electron, elements in groups 3-12 have a variable number of valence electrons, and elements in group 17 have 7 valence electrons. The lanthanides and actinides, located below the main table, all fit within group 3.
How does the atomic number of an element increase?
Element atomic number increases as you move down a group from top to bottom or across a period from left to right. An element group is a vertical column on the periodic table. Atoms in a group share the same number of valence electrons. An element period is a horizontal row on the periodic table. Atoms in a period have the same number ...
What are the different types of nonmetals?
The nonmetals, halogens, and noble gases are all types of nonmetals. The metalloids have properties intermediate between metals and nonmetals. The alkali metals, alkaline earths, lanthanides, actinides, transition metals, and basic metals are all groups of metals.
How many groups are there in the periodic table?
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells ...
What is the group 8 of elements?
An exception is the " iron group ", which usually refers to " group 8 ", but in chemistry may also mean iron, cobalt, and nickel, or some other set of elements with similar chemical properties. In astrophysics and nuclear physics, it usually refers to iron, cobalt, nickel , chromium, and manganese .
What group is the noble gases in?
b Group 18 , the noble gases, were not discovered at the time of Mendeleev's original table. Later (1902), Mendeleev accepted the evidence for their existence, and they could be placed in a new "group 0", consistently and without breaking the periodic table principle.
What is a group of elements made of hydrogen?
In history, several sets of group names have been used: a Group 1 is composed of hydrogen (H) and the alkali metals. Elements of the group have one s-electron in the outer electron shell. Hydrogen is not considered to be an alkali metal as it is not a metal, though it is more analogous to them than any other group.
When did group 1 and 18 change to group 18?
The modern numbering system of "group 1" to "group 18" has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) since about 1990. It replaces two older incompatible naming schemes, used by the Chemical Abstract Service (CAS, more popular in the US), and by IUPAC before 1990 (more popular in Europe).
Is hydrogen an alkali metal?
Hydrogen is not considered to be an alkali metal as it is not a metal, though it is more analogous to them than any other group. This makes the group somewhat exceptional. b Group 18, the noble gases, were not discovered at the time of Mendeleev's original table.
How many elements are in the periodic table?
The table below consists of 118 elements of the periodic table, sorted by atomic number, atomic weight, symbols, density, discovered year and the group.
Who created the modern periodic table?
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley, which states that “the properties of elements are periodic functions of their atomic numbers”.
What is the atomic number of an element?
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of [He] 2s 2 2p 2, since its atomic number is 6.
What is the number of protons in the nucleus called?
The number of protons in the nucleus is called the atomic number. The atomic number of each element is unique.
Why is the atomic number of each element unique?
While the atomic number always stays the same some elements have atoms with different atomic mass numbers. This is because some elements have a different number of neutrons in the nucleus.
How to find the mass of an element?
The number of protons and the number of neutrons shall determine the mass number of an element. Since the isotopes of an element have slightly different mass numbers, it calculates the atomic mass by obtaining the mean of the mass numbers for its isotopes.
How can periodic trends be observed?
Periodic trends in the properties of the elements can be observed down the groups and across the periods of the modern periodic table. Every chemical element has a specific atomic number, which provides insight into the number of protons present within its nucleus.
How does the periodic table organize the elements?
The classic Periodic Table organizes the chemical elements according to the number of protons that each has in its atomic nucleus. (Image credit: Karl Tate, Livescience.com contributor)
Which group of the periodic table is alkaline earth metals in?
Alkaline-earth metals: The alkaline-earth metals make up Group 2 of the periodic table, from beryllium (Be) through radium (Ra). Each of these elements has two electrons in its outermost energy level, which makes the alkaline earths reactive enough that they're rarely found alone in nature. But they're not as reactive as the alkali metals. Their chemical reactions typically occur more slowly and produce less heat compared to the alkali metals.
What are the groups of metals that are radioactive?
All are radioactive. The actinides and the lanthanides together form a group called the inner transition metals. Transition metals: Returning to the main body of the table, the remainder of Groups 3 through 12 represent the rest of the transition metals.
What are the elements in the actinides?
Actinides: The actinides line the bottom row of the island and comprise elements 89, actinium (Ac), through 103, lawrencium (Lr). Of these elements, only thorium (Th) and uranium (U) occur naturally on Earth in substantial amounts. All are radioactive. The actinides and the lanthanides together form a group called the inner transition metals.
What is table salt?
The table salt in your kitchen, for example, is a marriage between the alkali metal sodium and the halogen chlorine. Noble gases: Colorless, odorless and almost completely nonreactive, the inert, or noble gases round out the table in Group 18.
How many elements were there at the time of Mendeleev?
There were only about 60 elements known at the time, but Mendeleev realized that when the elements were organized by weight, certain types of elements occurred in regular intervals, or periods. Today, 150 years later, chemists officially recognize 118 elements (after the addition of four newcomers in 2016) and still use Mendeleev's periodic table ...
Why is the period of sodium longer?
Moving down the table, periods are longer because it takes more electrons to fill the larger and more complex outer levels. The columns of the table represent groups, or families, of elements.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
How many categories are there in the periodic table?
The elements of the periodic table shown here are divided into nine categories; six for the metals, and two for nonmetals, and a metalloid category. The nine categories (or sets) correspond to those found in the literature for the applicable part of the periodic table. Different authors may use different categorisation schema depending on the properties of interest.
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What is the electron configuration of a neutral atom?
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered 1, 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram. The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
How many electrons are in neon?
The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to ...
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
How many periods are there in the periodic table?
The periodic table of the elements contains all of the chemical elements that have been discovered or made; they are arranged, in the order of their atomic numbers, in seven horizontal periods, with the lanthanoids (lanthanum, 57, to lutetium, 71) and the actinoids (actinium, 89, to lawrencium, 103) indicated separately below.
What are the elements in the periodic table?
It is customary to refer to horizontal series of elements in the table as periods and vertical series as groups. The seven elements lithium to fluorine and the seven corresponding elements sodium to chlorine are placed in the seven groups, 1 (Ia), 2 (IIa), 13 (IIIa), 14 (IVa), 15 (Va), 16 (VIa), and 17 (VIIa), respectively. The 17 elements of the fourth period, from potassium, 19, to bromine, 35, are distinct in their properties and are considered to constitute Groups 1–17 (Ia–VIIa) of the periodic system.
What group of elements show a gradual increase in hardness and melting point?
The elements of the long periods show a gradual increase in hardness and melting point from the beginning alkali metals to near the centre of the period and then at Group 16 (VIa) an irregular decrease to the halogens and noble gases. The valence of the elements (that is, the number of bonds formed with a standard element) is closely correlated ...
What are the alkali metals?
The first group, the alkali metals, thereby includes, in addition to lithium and sodium, the metals from potassium down the table to francium but not the much less similar metals of Group 11 (Ib; copper, etc.). Also the second group, the alkaline-earth metals, is considered to include beryllium, magnesium, calcium, strontium, barium, ...
What is the valence of an element?
The valence of the elements (that is, the number of bonds formed with a standard element) is closely correlated with position in the periodic table, the elements in the main groups having maximum positive valence, or oxidation number, equal to the group number and maximum negative valence equal to the difference between eight and the group number.
Which element is the least electronegative?
It is shown to depend upon the element’s position in the periodic table in the same way that nonmetallic character does, fluorine being the most electronegative element and cesium (or francium) the least electronegative (most electropositive) element. The sizes of atoms of elements vary regularly throughout the periodic system.
What are the elements in the 18 (0) group?
The six noble gases—helium, neon , argon, krypton, xenon, and radon—occur at the ends of the six completed periods and constitute the Group 18 (0) group of the periodic system. It is customary to refer to horizontal series of elements in the table as periods and vertical series as groups. The seven elements lithium to fluorine and ...