)
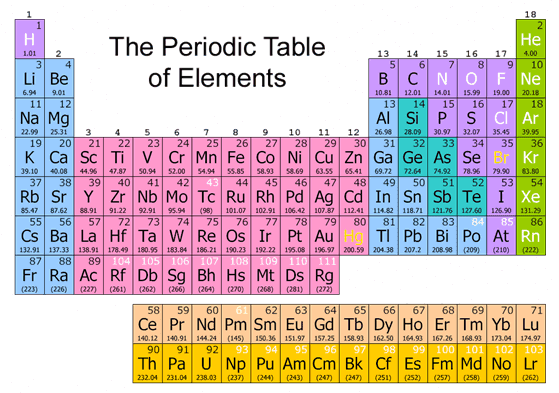
How does the periodic table work?
Here's how it works: Elements are listed in numerical order by atomic number. The atomic number is the number of protons in an atom of that element. So element number 1 (hydrogen) is the first element.
How many periods are there in the periodic table?
There are seven periods on the periodic table. Elements in the same period all have the same electron ground state energy level. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties.
What are the trends in the periodic table?
As you progress in chemistry, there are other trends in the periodic table you'll need to know: 1 Atomic radius and ionic radius increase as you move down a group, but decrease as you move across a period. 2 Electron affinity decreases as you move down a group, but increases as you move across a period until you get to the last column. The elements in this group, the noble gases, have practically no electron affinity. 3 The related property, electronegativity, decreases going down a group and increases across a period. Noble gases have practically zero electronegativity and electron affinity because they have complete outer electron shells. 4 Ionization energy decreases as you move down a group, but increases moving across a period. 5 Elements with the highest metallic character are located on the lower left side of the periodic table. Elements with the least metallic character (most nonmetallic) are on the upper right side of the table.
What are the two main types of elements?
The two main types of elements are metals and nonmetals . There are also elements with properties intermediate between metals and nonmetals. These elements are called metalloids or semimetals. Examples of groups of elements that are metals include alkali metals, alkaline earths, basic metals, and transition metals.
Why is the periodic table important?
The periodic table is one of the most valuable tools for chemists and other scientists because it orders the chemical elements in a useful way. Once you understand how the modern periodic table is organized, you'll be able to do much more than just look up element facts like their atomic numbers and symbols.
What is the element symbol?
The element symbol is a shorthand notation that is either one capital letter or a capital letter and a lowercase letter. The exception is the elements at the very end of the periodic table, which have placeholder names (until they are officially discovered and named) and three-letter symbols.
What is the atomic number of an element?
Every atom of hydrogen has 1 proton. Until a new element is discovered, the last element on the table is element number 118 . Every atom of element 118 has 118 protons.
How are the columns in the periodic table organized?
The rows and columns are organized by precise characteristics. The elements that are in the same column or in the same rows have common characteristics. For example, magnesium (Mg) and sodium ...
What is the periodic table called?
Let us investigate periods. After all, that is how the periodic table gets its name. Each of the rows from left to right is called a period. What that means in that each and every one of the elements in a row shares similar electron configurations with the others. Or, in other words, each of the elements in the same row has the exact same number of atomic orbitals.
How many electrons does helium have?
Helium (He) is unique among all the elements. It only has two electrons in its outer orbital, also known as the valence shell. All the other noble gases (group 18) have eight electrons in their outer orbital or valence shell.
How many orbitals does an element have?
If you look at all the elements on the top row or, in other words, the elements in the first period, you will see that all of them have one atomic orbital for their electrons. Then, the elements on the second row, or second period, are characterized by having two atomic orbitals in their electrons.
How to read valence electrons?
You have to read groups from left to right. All the elements in the first column, or group one, have one valence electrons (one electron in their outer shell). All the elements in the second column, or group two, have two valence electrons. But all the elements in the third group (group three), have thirteen valance electrons. From then on, you have to add an electron for every group until reaching 18. Simply, counting the columns will allow you to know how many electrons each element has on its outer shell. There are a few exceptions to this, though, because some elements are transition elements that add electrons.
Why do all elements have one thing in common?
Because they all have one thing in common: their respective valence shells are full. This is how the periodic table is organized. Understanding that the position of each and every one of the elements is useful in understanding their properties.
How many valance electrons are in the third group?
But all the elements in the third group (group three), have thirteen valance electrons. From then on, you have to add an electron for every group until reaching 18. Simply, counting the columns will allow you to know how many electrons each element has on its outer shell.
Groups of the Periodic table
Alkali metals: The alkali metals make up most of Group 1, the table's first column. Shiny and soft enough to cut with a knife, these metals start with lithium (Li) and end with francium (Fr). They are also extremely reactive and will burst into flame or even explode on contact with water, so chemists store them in oils or inert gases.
Additional resources
Watch this brief video about the periodic table and element groups, from Crash Course.