Where is number of neutrons located in periodic table?
The number of neutrons can be calculated by simply looking at the Periodic Table of Elements. The number of neutrons= mass number – atomic number Each element in the table has the mass number (atomic weight) located directly under the Element name and the atomic number is located at the top left hand corner of an element in the table.
How can you find valence electrons on the periodic table?
Remember to follow these steps so that you can find the right valence electrons:
- You can refer to a periodic table of elements.
- Look at the different columns that are available, and you will see that there are 18 that you can find right now.
- Take note that all of the elements that are under one vertical column will have the same number of valence electrons.
Does the periodic table tell the number of protons?
The periodic table tells us: Mass. Common charge. Number of Protons, Neutrons, and Electrons. Class/Period (reaction characteristics and element properties) Valence Electrons. Group. What can you learn from periodic table? The periodic table of elements puts all the known elements into groups with similar properties.
How do you find number of neutrons in an element?
number of neutrons = mass number - atomic number. Also question is, how do you find the protons neutrons and electrons of an element? Explanation: You can simply subtract the atomic number from the mass number in order to find the number of neutrons. If the atom is neutral, the number of electrons will be equal to the number of protons.
How do you find neutrons from protons?
4:3113:12How To Calculate The Number of Protons, Neutrons, and ElectronsYouTubeStart of suggested clipEnd of suggested clipThe number of neutrons is the difference between the mass number which is 23 and the atomic numberMoreThe number of neutrons is the difference between the mass number which is 23 and the atomic number which is 11.. So it's 23 minus 11. And that gives us 12.
Where can you find the neutrons of an atom?
the nucleusProtons and neutrons are found in the nucleus. They group together in the center of the atom.
How do you find neutrons without atomic mass?
Subtract the atomic number from the atomic weight and you will get the atomic mass or the number of neutrons of that element.
How many neutrons are in an atom?
Its atomic number is 14 and its atomic mass is 28. The most common isotope of uranium has 92 protons and 146 neutrons. Its atomic number is 92 and its atomic mass is 238 (92 + 146)....2.1 Electrons, Protons, Neutrons, and Atoms.Elementary ParticleChargeMassNeutron01Electron−1~01 more row
How do you find the protons electrons and neutrons?
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number: number of protons = atomic number. number of electrons = atomic number. number of neutrons = mass number - atomic number.
Are protons and neutrons equal?
Number of neutrons is always greater than or equal to the number of protons. Number of neutrons is always greater than or equal to the number of protons.
How do you find electrons on the periodic table?
Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number.
How do you get the number of neutrons in a nucleus?
To calculate the number of neutrons, subtract the atomic number (which equals the number of protons) from the mass number.
How do you find protons on the periodic table?
0:022:13Calculating the Protons, Neutrons and Electrons for an Atom - YouTubeYouTubeStart of suggested clipEnd of suggested clipOkay you should be familiar with the two numbers on the periodic table the top one being the massMoreOkay you should be familiar with the two numbers on the periodic table the top one being the mass number which is the number of protons. And neutrons add it together.
Are neutrons and electrons the same?
Electrons are a type of subatomic particle with a negative charge. Protons are a type of subatomic particle with a positive charge. Protons are bound together in an atom's nucleus as a result of the strong nuclear force. Neutrons are a type of subatomic particle with no charge (they are neutral).
Is the total number of protons and neutrons?
mass numberThe mass number of an atom is the total number of protons and neutrons in its nucleus. Atoms of different elements usually have different mass numbers, but they can be the same.
Why do elements have neutrons?
Neutrons are required for the stability of nuclei, with the exception of the single-proton hydrogen nucleus. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
Where are electrons found in an atom?
Protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus.
How do we determine the number of neutrons from A and Z?
To determine the number of neutrons in an element you subtract the atomic number from the atomic mass of the element. Atomic mass is represented as (A) and atomic number is represented as (Z) and neutrons are represented as (N).
How do you find the number of neutrons in an isotope?
To find the number of neutrons in an isotope, subtract the number of protons from the atomic mass of the isotope. The atomic number of the element equals the number of protons.
How can you calculate the number of neutrons in an atom gizmo?
To calculate the number of neutrons in the nucleus of an atom is simple. You take the atomic, or proton number of the element, and you subtract it from the element's mass number.
How to find the number of neutrons in an atom?
The formula for finding out the number of neutrons in an atom is atomic mass – atomic number. The intuition behind this hinges on how the atomic mass presented on the periodic table is calculated. The atomic mass of an atom is determined by only the number of protons and neutrons.
Where is the atomic number of neutrons?
Each element in the table has the mass number (atomic weight) located directly under the Element name and the atomic number is located at the top left hand corner of an element in the table. Don’t forget to round the mass number (atomic weight) to the nearest whole number.
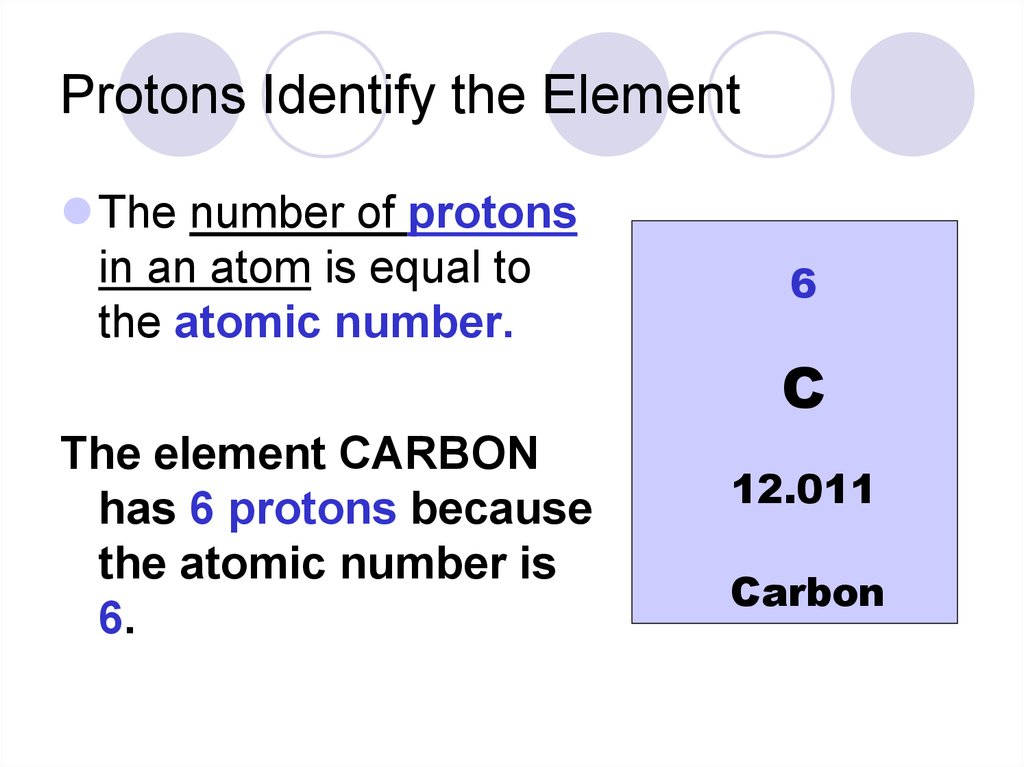
How many neutrons are in carbon?
For example, Carbon’s atomic number/number of protons is 6 and the mass number is 12.011. This means there are 6 neutrons (approximately). The number of neutrons can be calculated by simply looking at the Periodic Table of Elements. The number of neutrons= mass number – atomic number.
What is the atomic number of an element on the periodic table?
You also have to know that the atomic number of an element on the periodic table to equal to the number of protons that atom contains. It then makes perfect sense why the number of neutrons is equal to the atomic mass (protons + neutrons) minus the atomic number (protons only).
What is the atomic number of an element?
The mass number is the sum of protons and neutrons in the nucleus. The atomic number is the number of protons.
How to find neutrons in a periodic table?
To find the neutrons in an element of the periodic table, you need two things, the atomic number of the element and the atomic mass of that element. See below
Why does the atomic mass of an element not include electrons?
Right below the chemical symbol, you will find a number that usually has decimals and is not a whole number...this is the atomic mass of the element and it represents the number of protons and neutrons in the element, the reason why it does not also account for the electrons is because the electron mass is negligible ....
What is the atomic number of an atom?
The atomic number is the number of protons OR electrons. They are both equal thus making the atom have a neutral charge.
How to find neutrons?
Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. Remember that the atomic number is the same as the number of protons, which you have already identified.
What is the periodic table?
The periodic table is a chart that organizes elements by their atomic structure. It is color-coded and assigns each element a unique 1 or 2-letter abbreviation. Other elemental information includes atomic weight and atomic number. You can find a periodic table online or in a chemistry book.
Why is an ion positive?
Although the number of protons in the atom remains the same, the number of electrons is altered in an ion. Because an electron has a negative charge, when you remove electrons, the ion becomes positive.
Why does an ion become positive when you remove electrons?
Because an electron has a negative charge, when you remove electrons, the ion becomes positive. When you add more electrons, the ion becomes negative.
Where is the atomic number located?
The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element. For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
What are the three groups of elements?
Find your element on the periodic table. The table orders elements by atomic number and separates them into three main groups: metals, non-metals, and metalloids (semi-metals). Further elemental groupings include alkali metals, halogens, and noble gases.
Which particles have a positive charge equal to +1?
Protons are particles in the nucleus of an atom that have a positive charge equal to +1. Electrons are particles that have a negative charge equal to -1. Therefore, an element in a neutral state will have the same number of protons and electrons.
How to find the number of neutrons in an atom?
Categories: Chemistry. Article Summary X. To find the number of neutrons in an atom, start by locating the element on the periodic table. Then, find the element's atom number, which is usually in the top left or middle of the box above the element symbol.
What is the atomic number of a periodic table?
If you’re ever unsure which number is which in a periodic table, just remember that the table is usually designed around the atomic number (i.e. number of protons), which starts at 1 (hydrogen) and ascends one unit at a time from left to right, ending at 118 (oganesson).
What is the empty space between the atomic cloud of an atom and its nucleus?
The empty space between the atomic cloud of an atom and its nucleus is just that: empty space, or vacuum. That's the simple answer, but there are a few subtleties: Subatomic particles such as electrons, protons and neutrons need to be treated as quantum objects.
How to find the atomic mass of an atom?
Add the number of protons (also known as atomic number) and neutrons in a single atom. The atomic mass can also be located in the same square as the desired element on the periodic table. For example, the atomic mass of scandium (Sc) would be 44.96.
What is the atomic number of an element?
The atomic number is the number of protons in a single atom of that element. Os is number 76, meaning one atom of osmium has 76 protons. The proton number never changes in an element; it's basically what makes that element that element.
What is the number of protons in the nucleus of an atom?
The number of protons in the nucleus of the atom is equal to the chemical's atomic number.
How to tell if an atom is an isotope?
Although all atoms of the same element contain the same number of protons, their number of neutrons can vary. Knowing how many neutrons are in a particular atom can help you determine if it's a regular atom of that element or an isotope, which will have either extra or fewer neutrons. Determining the number of neutrons in an atom is fairly simple ...