How to Find Electrons on the Periodic Table
- Obtain a periodic table of elements.
- Find the element in question on the periodic table.
- Find the atomic number of an element.
- Identify the charge of the ion.
- Subtract the charge from the atomic number if the ion is positive.
- ... (more items)
Which is more electronegative n or O?
The most electronegative component is Fluorine with a rating of 4.0. Across from Fluorine we also have N(Nitrogen) and O (Oxygen)with high electronegativities. Electronegativity is essentially how many elements ‘want’ electrons. An easy way to think about it is that the closer an element is to Fluorine, the more powerful its electronegativity is.
How to determine the electrons?
Step 3: Determine the number of electrons:
- If it is a neutral atom, the number of electrons is equal to the proton number
- If the ion is positively charged, the number of electrons is found by subtracting the charge number from the proton number.
- If the ion is negatively charged, the number of electrons is found by adding the charge number to the proton number.
How many electrons are in each shell including 3p orbitals?
The p sublevel has 3 orbitals, so can contain 6 electrons max. The d sublevel has 5 orbitals, so can contain 10 electrons max. What is the maximum number of electrons in the first orbit? Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on.
What is the electronegativity on the periodic table?
What Is Electronegativity and How Does It Work?
- Electronegativity Example. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the Cl than to the H in the HCl ...
- Most and Least Electronegative Elements. The most electronegative element on the periodic table is fluorine (3.98). ...
- Electronegativity as a Periodic Table Trend. ...
- Sources. ...
How do you read protons neutrons and electrons on the periodic table?
0:004:23How to find the Protons Neutrons and Electrons of an element ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipWelcome to moomoomath and science in this video let's learn to use the periodic table in order toMoreWelcome to moomoomath and science in this video let's learn to use the periodic table in order to find an elements name symbol number of protons neutrons. And electrons take a look at this chart.
How do you identify distinguishing electrons?
The value of n, the principal quantum number for the distinguishing electron, can be quickly determined by counting down from the top of the periodic table. For example, iodine is a representative element in the fifth period. Therefore the distinguishing electron must occupy either the 5s or 5p subshell.
How do you determine an element's electrons and neutrons?
An element is defined by its number of protons. Varying the number of neutrons will change the isotope of an element and varying the number of electrons will create an ion, but changing the number of protons changes the identity of the element itself.
What is electron in periodic table?
Electron configurations and the periodic table. Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number.
What are the valence electrons on the periodic table?
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴.
How do you find the number of electrons in a molecule?
0:000:54How to calculate number of electrons for ions in less than 60s - Dr KYouTubeStart of suggested clipEnd of suggested clipHere's a simple trick to count the number of electrons in ions all we have to do is take the numberMoreHere's a simple trick to count the number of electrons in ions all we have to do is take the number of electrons in the atoms. And subtract its charge. This simple trick works for both cation.
Are protons and electrons equal?
The number of electrons on a neutral atom is equal to the number of protons in the nucleus of the atom. This is known as the atomic number, Z. The removal or addition of electrons to a neutral atom creates ions that have a net negative or positive charge.
What is the formula for number of electrons?
The formula used for calculating the number of electrons will be q=ne . Additional information Electric charge is the amount of energy or electrons that pass from one body to another by different modes like conduction, induction etc.
How do you determine the identity of an element?
The identity of an element is determined by the number of protons in the nucleus of one of its atoms, which is also the atomic number for that element.
How do you find electrons in an ion?
0:000:54How to calculate number of electrons for ions in less than 60s - Dr KYouTubeStart of suggested clipEnd of suggested clipHere's a simple trick to count the number of electrons in ions all we have to do is take the numberMoreHere's a simple trick to count the number of electrons in ions all we have to do is take the number of electrons in the atoms. And subtract its charge. This simple trick works for both cation.
What distinguishes a neutral atom from an ion?
Atoms are neutral; they contain the same number of protons as electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
How do you find protons neutrons and electrons in an ion?
0:596:32Finding the Protons, Neutrons, Electrons, & Mass Number for IonsYouTubeStart of suggested clipEnd of suggested clipSo with n a plus the sodium cation. We've lost an electron. So instead of 11 we now have 10. TheMoreSo with n a plus the sodium cation. We've lost an electron. So instead of 11 we now have 10. The mass number that is the protons plus neutrons 11 plus 12 gives us 13..
How are electrons arranged around the nucleus of an atom?
Just like the moon revolving around the earth, the electrons also revolve around the nucleus.
How many electrons can a shell hold?
1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now I’ll show you the complete list of elements with electrons per shell.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
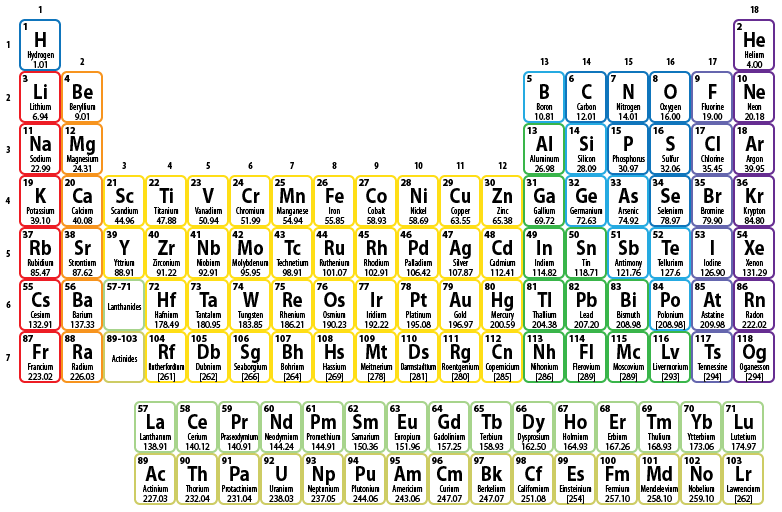
What is the periodic table?
The periodic table is a chart that organizes elements by their atomic structure. It is color-coded and assigns each element a unique 1 or 2-letter abbreviation. Other elemental information includes atomic weight and atomic number. You can find a periodic table online or in a chemistry book.
Where is the atomic number located?
The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element. For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
Why is an ion positive?
Although the number of protons in the atom remains the same, the number of electrons is altered in an ion. Because an electron has a negative charge, when you remove electrons, the ion becomes positive.
How to find neutrons?
Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. Remember that the atomic number is the same as the number of protons, which you have already identified.
Why does an ion become positive when you remove electrons?
Because an electron has a negative charge, when you remove electrons, the ion becomes positive. When you add more electrons, the ion becomes negative.
What are the three groups of elements?
Find your element on the periodic table. The table orders elements by atomic number and separates them into three main groups: metals, non-metals, and metalloids (semi-metals). Further elemental groupings include alkali metals, halogens, and noble gases.
Which type of charge is positively charged?
Protons are positively charged, electrons are negatively charged, and neutrons have no (neutral) charge.
Where is the letter symbol on the periodic table?
Locate the desired element on the periodic table. Each square on the periodic table contains the letter symbol for an element printed directly below the atomic number of the element.
What are the two lines of elements on the periodic table?
There are two lines of elements listed below the main table on the periodic chart, the lanthanides and actinides. All lanthanides belong in Period 6, Group 3. Actinides belong in Period 7, Group 3. These elements are known as inner transition metals.
How do valence electrons affect the chemical reaction?
Atoms tend to accept or lose electrons if doing so will result in a full outer shell. Accordingly, valence electrons directly influence how elements behave in a chemical reaction.
How to determine if an element is not a transition metal?
The rule is as follows: If an element is not a transition metal, then valence electrons increase in number as you count groups left to right, along a period. Each new period begins with one valence electron. Exclude groups 3 through 12. These are transitional metals, which have special circumstances.
How many valence electrons does oxygen have?
Oxygen is located in group 16 on the periodic table, so it has six valence electrons.
How many apparent valence electrons does iron have?
The element iron is in group 8, and therefore has two or three apparent valence electrons.
How many electrons are in an electron shell?
Electron shells are labeled K, L, M, N, O, P, and Q or simply 1 to 7; starting with the shell closest to the nucleus and moving out. Each electron shell can hold a fixed, maximum number of electrons: the K shell holds a maximum of two electrons, the L shell holds eight electrons, the M shell holds eighteen electrons and the N shell holds a maximum of thirty-two electrons. Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell.
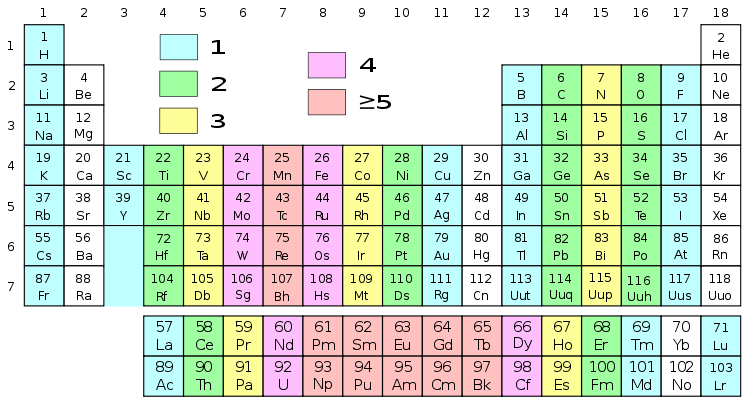
How to find valence electrons in a period table?
To find valence electrons using a period table, first see if your atom is a transitional metal, which are the elements in the middle rectangle of the table. If the atom is outside this block, locate its group number along the top of the table. The ones digit in the group number is the number of valence electrons.
How to label a column on the periodic table?
Label each column on the periodic table of elements from 1 to 18. Generally, on a periodic table, all of the elements in a single vertical column will have the same number of valence electrons. If your periodic table doesn't already have each column numbered, give each a number starting with 1 for the far left end and 18 for the far right end. In scientific terms, these columns are called the element "groups."
How to find the number of valence electrons in an atom?
Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones place of the group number is the number of valence electrons in an atom of these elements. In other words:
How many electrons does sodium have?
So, for our example, we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital. That's 11 electrons total — sodium is element number 11, so this makes sense.
How many valence electrons does carbon have?
In our example, since carbon is in group 14, we can say that one atom of carbon has four valence electrons.
How to determine the number of valence electrons?
Determine the number of valence electrons based on the group number. Once again, the group number of the element you are examining can tell you its valence electrons. However, for the transition metals, there isn't a pattern you can follow — group number will usually correspond to a range of possible numbers of valence electrons. These are:
How many orbital shells does Selenium have?
For example, we know the element selenium has four orbital shells because it is in the fourth period. Since it is the sixth element from the left in the fourth period (ignoring the transition metals), we know that the outer fourth shell has six electrons, and, thus, that Selenium has six valence electrons.
Where is the atomic number on the periodic table?
The atomic number is usually located in the top left hand corner of the box on the periodic table, and the atomic weight is located directly under the element name. Round the atomic weight to the nearest whole number. Gold has an atomic number of 79 and an atomic weight of 196.966569, or 197.
What is the periodic table?
The periodic table lists every element on Earth and information about those elements. With this table, you can see how the elements relate to each other and how to find out how many particles are in an atom of each of them. An atom is made up of protons, electrons and neutrons.
How to find the atomic number of gold?
Gold has an atomic number of 79 and an atomic weight of 196.966569, or 197. Calculate the number of neutrons by subtracting the atomic number from the atomic weight. The atomic number is equal to the number of protons in an atom. The atomic weight is equal to the total number of particles in the atom's nucleus.
How to find the number of neutrons in an atom?
Calculate the number of neutrons by subtracting the atomic number from the atomic weight. The atomic number is equal to the number of protons in an atom. The atomic weight is equal to the total number of particles in the atom's nucleus. Since protons and neutrons occupy the nucleus together, subtracting the number of protons from the total particles will give you the number of neutrons. (For gold: 197 - 79 = 118 neutrons)
How many neutrons are in gold?
Since protons and neutrons occupy the nucleus together, subtracting the number of protons from the total particles will give you the number of neutrons. (For gold: 197 - 79 = 118 neutrons) Things You'll Need. Periodic table.
What are atoms made of?
An atom is made up of protons, electrons and neutrons. Choose an element and find it on the periodic chart. For this example, use gold, which is located in row six of the table (atomic sign: Au). Locate the atomic number and the atomic weight of the element.
