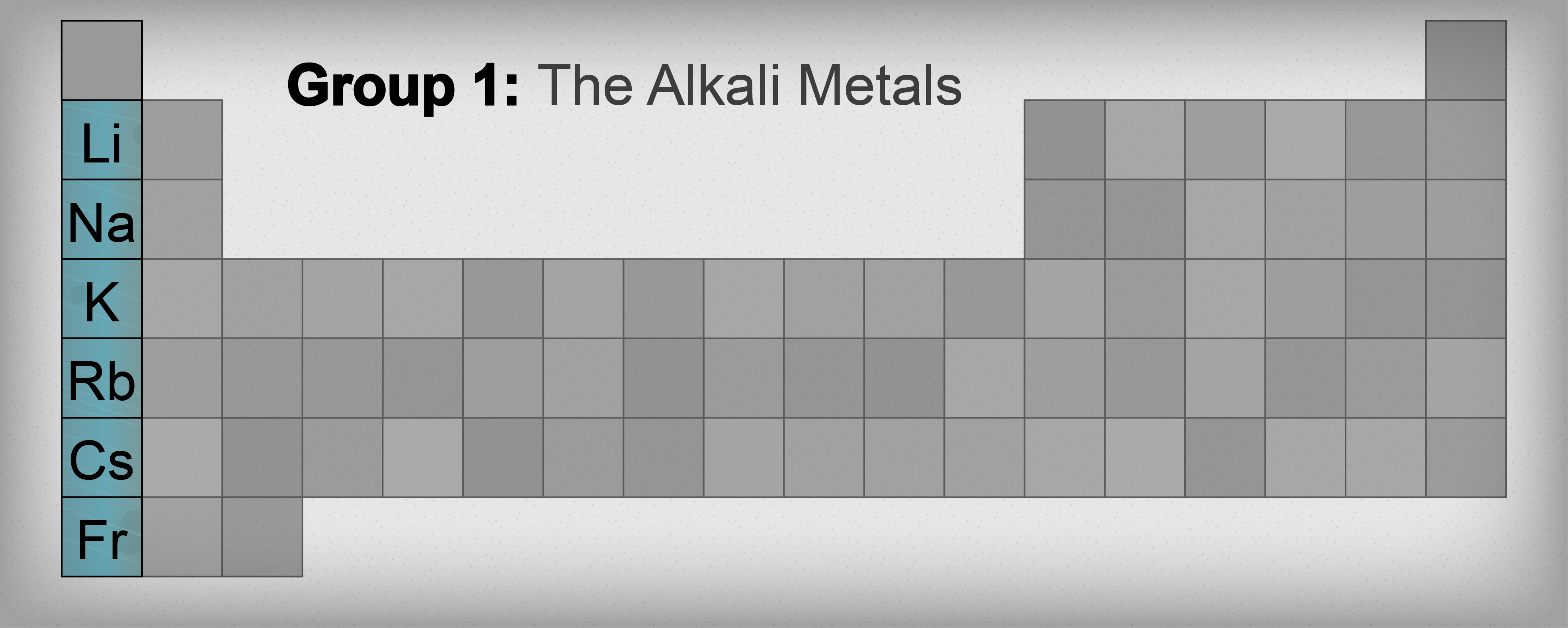
Alkali metal
The alkali metals are a group (column) in the periodic table consisting of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). This group lies in the s-block of the periodic table of elements as all alkali metals have their outermost electron …
Full Answer
What are the alkaline metals on the periodic table?
Here are a few alkaline earth metal electron configurations:
- Beryllium: [He]2s 2
- Magnesium: [Ne]3s 2
- Strontium: [Kr]5s 2
What are 3 facts about alkali metals?
Alkali metals have one electron in their outer shell, which is loosely bound. This gives them the largest atomic radii of the elements in their respective periods. Their low ionization energies result in their metallic properties and high reactivities. An alkali metal can easily lose its valence electron to form the univalent cation. Alkali metals have low electronegativities. They react readily with nonmetals, particularly halogens.
How are metals classified in the periodic table?
Types of Metals in the Periodic Table
- Alkali Metals. Alkali metals are present at the top left of the periodic table. ...
- Alkaline Earth Metals. Alkaline earth metals are also present at the top left of the periodic table. ...
- Transition Metals. Transition metals are present in the middle of the periodic table. ...
- Lanthanides. Lanthanides are also found in the middle of the periodic table. ...
- Actinides. ...
What elements are in alkali metals?
Alkali metals are the six elements that comprise Group I in the Periodic Table: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Especially when dissolved in water, these elements form strong bases (alkalis) capable of reacting with and neutralizing strong acids.

What does alkali metals mean in the periodic table?
alkali metal, any of the six chemical elements that make up Group 1 (Ia) of the periodic table—namely, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). The alkali metals are so called because reaction with water forms alkalies (i.e., strong bases capable of neutralizing acids).
Where are alkali metals on the periodic table?
Group 1AGroup 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These are (except for hydrogen) soft, shiny, low-melting, highly reactive metals, which tarnish when exposed to air.
What makes an alkali metal?
Alkali metals all have one electron in their outer layer and are found in column 1A of the periodic table. Usually found as ionic compounds or as ions, they readily give up their electron to other elements.
What are alkali metals and their uses?
Uses of alkali metalsPure sodium has many applications, including use in sodium-vapor lamps which produce very efficient light .Potassium has a vital rolel in biological system. KCl used as a fertilizer while KOH is used in the manufacture of soap.Caesium is used making photoelectric cells.
What are 3 facts about alkali metals?
Alkali metals share many similar properties including:They are shiny, soft, metals.They are very reactive.They all have one valence electron in the outermost shell which they seek to lose in order to have a full outer shell. ... They are soft enough to be cut with a knife.More items...
What are three characteristics of alkali metals?
General Characteristics of Alkali MetalsAlkali metals are soft metals due to weak metallic bonding. ... They have low melting and boiling points.They are highly reactive metals. ... The energy required to remove an electron from the outermost shell is known as Ionization Energy. ... Sodium is the most abundant metal.More items...
Why are alkali metals so important?
Alkali metals, particularly sodium, are important in commercial use and chemical synthesis. Alkali metals are highly conductive soft metals, which have a high lustre that oxidizes quickly when they are exposed to air. The Alkali metals are the most reactive metals in the periodic table.
Why are alkali important?
Alkaline diets also tend to be low in fat and calories, naturally promoting a healthy body weight and lowering heart disease risk factors. They also reduce or eliminate red and processed meats, removing a major contributor to heart disease from the diet.
What are alkali metal give example?
Alkali metals have a corresponding [Noble gas] ns1 electronic configuration. They occupy the first column of the periodic table. Alkali elements are Lithium(Li), Sodium(Na), Potassium (K), Rubidium (Ru), Cesium (Cs) and Francium (Fr) occupying successive periods from first to seven.
What are 5 characteristics of alkali metals?
Characteristics of alkali metals are:High reactive metals.Not found freely in nature.Stored in a mineral oil solution.Low melting points.Low densities (lower than other metals)Low electronegativity.Low ionization energy.React easily with halogens.More items...
What are 2 characteristics of alkali earth metals?
Alkaline earth metals have two valence electrons. They are highly reactive and are not found naturally in their elemental state. The two valence electrons are easily removed to form divalent cations. Alkaline earth metals are good conductors of electricity.
Which group of periodic table is alkali metals?
Alkali metals are present in the first group of Periodic table. First group indicates that they have only one electron in their outermost orbit. As only one electron is present in the outermost orbit, it is very easy to lose or donate this electron during a chemical reaction.
How many electrons are in an alkali metal?
Look, as we move down the group from top to bottom, the atomic size increases. Also alkali metals have only one electron in their outermost orbit.
Why are alkali metals so reactive?
There are many reasons behind the reactivity of alkali metals. Let us discuss them one by one.
What metals react with water?
When these metals (Li, Na, K, Rb, Cs, Fr) react with water, they form alkalis (i.e strong base).
Which element is the most reactive?
And hence the element with bigger atomic size (i.e Francium) is most reactive alkali metal. ( Note: Francium is a laboratory made element. It is available in a very less quantity. And hence for any practical purposes, cesium is considered as the most reactive metal in Periodic table.
Where are alkali metals located?
Alkali metals are located in group 1 on the left side of the Periodic table.
Which metal decreases from top to bottom in a group?
Electronegativity of the alkali metals decreases from top to bottom in a group.
Which element is an alkali metal?
They occupy the first column of the periodic table. Alkali elements are Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Ru), Cesium (Cs) and Francium (Fr) occupying successive periods from first to seven. Francium is a radioactive element with very low half-life.
What are Alkali Metals?
In general ‘alkali’ refers to the basic or alkaline nature of their metal hydroxides. The compounds are called alkali metals because when they react with water they usually form alkalies which are nothing but strong bases that can easily neutralize acids.
How many electrons are in an alkali metal?
Alkali metals have one electron in their valence shell. The electronic configuration is given by ns 1 . For example, the electronic configuration of lithium is given by 1ns 1 2ns 1. They tend to lose the outer shell electron to form cations with charge +1 (monovalent ions).
What metals react with oxygen?
Alkali metals react with atmospheric oxygen and get tarnished of their shining nature. They burn with oxygen to form oxides. But, the nature of oxides formed is different.
Why is hydrogen not considered an alkali metal?
However, the main reason why hydrogen (H) is not considered as an alkali metal is that it is mostly found as a gas when the temperature and pressure are normal. Hydrogen can show properties or transform into an alkali metal when it is exposed to extremely high pressure.
Which side of the periodic table is alkali metal?
Alkali metals belong to the s-block elements occupying the leftmost side of the periodic table. Alkali metals readily lose electrons, making them count among the most reactive elements on earth. In this article, we will explain the electronic configurations, ionization enthalpy, hydration enthalpy and atomic, ionic radii and other physical ...
How are sodium and potassium obtained?
Hence, Sodium and potassium are obtained only by the electrolysis of the fused salts of sodium hydroxide and sodium chloride. Alkali metals form alloys with themselves, other metals, and amalgams with mercury.
What group are alkali metals in?
Location of the Alkali Metals on the Periodic Table. The alkali metals are the elements located in Group IA of the periodic table. The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium.
How many electrons are in an alkali metal?
Alkali metals have one electron in their outer shell, which is loosely bound. This gives them the largest atomic radii of the elements in their respective periods. Their low ionization energies result in their metallic properties and high reactivities.
What are batteries made of?
Household batteries are typically made of Lithium, an alkali metal. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. Learn about the properties of the alkali metals, one of the element groups.
What is an alkali metal?
Alkali metals are highly reactive chemical species that easily lose a single electron and form ionic compounds with non-metals.
Why do alkali metals not occur naturally?
The alkali metals, due to their high reactivity, they do not occur naturally in pure form in nature.
How is alkali metal halide formed?
Preparation– Alkali metal halide is formed by the reaction of oxides, hydroxides or carbonate with aqueous hydrohalic acid.
How are salts formed?
3) Salts of oxo acids: Salt are formed by the reaction of alkali metals with oxo acids. Alkali metal are high electropositive and hydroxide are strong base.
What is the oxidation state of alkali metal?
Oxidation state of alkali metal is +1. It is unipositive ion, positive charge of these elements increases down to group.
What is the measure of the tendency of an element to lose an electron?
Electrode potential measure tendency of element to lose electron.
What is the tendency of losing an electron to another element?
Tendency of losing an electron to another element is known as reducing capacity and that element is called as reducing agent.
How many valence electrons does an alkaline metal have?
Alkaline earth metals are similar in the following manner. All the alkaline earth metals have 2 valence electrons in their outermost orbit. They lose these two electrons and form cation with +2 charge. As you can see above that the alkaline earth metals have 2 electrons in the outermost shell.
What is the name of the metal that does not form an alkaline solution?
See the bold word “alkaline” and “earth” in the above 2 points, you will get the exact reason why they are called alkaline earth metals. ( Note: Beryllium (Be) is the only alkaline earth metal which does not form an alkaline solution on reacting with water.
What metals react with water?
They also react with water and moisture of the air, if they are kept open. As alkaline earth metals are reactive, they always occur in the compound form in nature. For example; Beryllium is found in its mineral beryl, chrysoberyl and phenacite. Magnesium is found in its mineral magnesite.
What happens to the metallic character of alkaline earth metals as the atomic size increases?
Down the group, the atomic size increases. And as the atomic size increases, the electron will be lost easily. So the metallic character of alkaline earth metals increases from top to bottom in a group. Read more about: Metallic character trend in periodic table.
What is the color of alkaline earth metals?
All the alkaline earth metals are silvery-white, shiny and somewhat reactive.
Where is magnesium found?
Magnesium is found in its mineral magnesite.
Is magnesium a metal?
Also, magnesium is obtained from the mineral magnesite which is mostly found from the earth crust. So magnesium is alka line earth metal. Calcium reacts with water and forms calcium hydroxide which is alkaline in nature.
What Are Alkali Metals on The Periodic table?
Are Alkali Metals Reactive?
- Yes, alkali metals are highly reactive. When alkali metals are exposed to water, they instantly react with water to form alkalis (strong base) and thereby hydrogen gas is produced.
Which Alkali Metal Is Most Reactive?
- Francium (Fr) is the most reactive alkali metal on the Periodic table. Here is an animation showing the reactivity of lithium, sodium and potassium with water. Reason? Look, as we move down the group from top to bottom, the atomic size increases. Also alkali metals have only one electron in their outermost orbit. Thus as the size of an atom increases, the loss of electron becomes very e…
List of Alkali Metals
- Here is a complete list of alkali metals present on the Periodic table with their atomic number, symbol and name of element.
Periodic Trends of Alkali Metals
- Valency As we move down the group, the valence of the elements remains the same. Read more about: Valency and its periodic trends Atomic size As we move down the group from top to bottom, the atomic size of the elements increases. Read more about: Atomic size and its periodic trends Metallic character Down the group, the atomic size increases. And as the atomic size incr…
Free Gift For You: Interactive Periodic Table
- Let me tell you how this Interactive Periodic Tablewill help you in your studies. 1).You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).You will get the detailed information about the periodic table which will convert a newbie into pro. 3).You will also get the HD images of the Periodic table (for FREE). Checkout Interactive Per…
Summary
- In the beginning of this article, I showed you a single image which clearly shows you where are Alkali metals located on the Periodic table. They are on the left side of the Periodic table(in group 1). Then we discussed about; 1. Meaning of alkali metals 2. Reactivity of alkali metals 3. Why are alkali metals so reactive? 4. Most reactive alkali metal 5. List of alkali metals 6. Electronic config…