What are periodic trends in chemistry?
Periodic Trends
- Variation of Physical Properties Within a Group. The physical properties (notably, melting and boiling points) of the elements in a given group vary as you move down the table.
- Electron Configurations and Magnetic Properties of Ions. ...
- Atomic Radius. ...
- Ionic Radius. ...
- Ionization Energy. ...
- Electron Affinity. ...
What is periodicity best defined as in chemistry?
Chemistry, 18.12.2019 03:31, miya763. Periodicity is best defined as: the fact that elements increase in atomic mass in a regular way the repeating nature of physical and chemical properties with atomic numbe the regular growth of atomic size with atomic mass
What is the early form of Chemistry?
noun. 1. 0. An early, unscientific form of chemistry practiced in the Middle Ages with aims including turning base metals into gold and discovering the elixir of perpetual youth, a universal cure for disease, and a universal solvent. Many alchemists were intelligent, well-meaning men and even distinguished scientists.
What does periodicity mean in chemistry?
Periodicity Definition In the context of chemistry and the periodic table, periodicity refers to trends or recurring variations in element properties with increasing atomic number. Periodicity is caused by regular and predictable variations in element atomic structure.

What is period and group?
A period is a horizontal row of the periodic table. A group is a vertical row of the periodic table.
What is a period in chemistry quizlet?
A period of elements on the periodic table is a horizontal row in which all the elements in that row have valence electrons in the same energy level.
What is the difference between a period and a group on the periodic table quizlet?
A horizontal row is called a period. A vertical column is called a group. What property of elements was used to organize the periodic table? The periodic table is organized in order of increasing atomic number.
What is a group on the periodic table quizlet?
a group in the periodic table consisting of five chemically related elements. metalloids. a chemical element with properties in between, or that are a mixture of, those of metals and nonmetals. metals.
What element is in period 4 group 15?
Arsenic. Arsenic (As) is an element in group 15. Arsenic, as mentioned above, is often used in semiconductors in alloys with germanium. Arsenic, in pure form and some alloys, is incredibly poisonous to all multicellular life, and as such is a common component in pesticides.
What element is in Period 6 Group 18?
Group 18 elements are the noble gases (inert gases or rare gases) He, Ne, Ar, Kr, Xe, Rn-222. They are colourless, odourless, monatomic gases and they form very few compounds.
What element is in Period 6 Group 10?
Group 10, numbered by current IUPAC style, is the group of chemical elements in the periodic table that consists of nickel (Ni), palladium (Pd), platinum (Pt), and perhaps also the chemically uncharacterized darmstadtium (Ds).
Which element is in period 5 Group 8?
Group 8 is a group (column) of chemical elements in the periodic table. It consists of iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs). They are all transition metals.
What is a period in the periodic table?
In the periodic table of the elements, each numbered row is a period. A period in the periodic table is a row of chemical elements. All elements in a row have the same number of electron shells. Each next element in a period has one more proton and is less metallic than its predecessor. Arranged this way, groups of elements in ...
Which period contains fewer elements than any other?
Period 1. The first period contains fewer elements than any other, with only two, hydrogen and helium. They therefore do not follow the octet rule, but rather a duplet rule. Chemically, helium behaves like a noble gas, and thus is taken to be part of the group 18 elements.
What are the elements that make up the basis of all organic compounds?
All organic compounds, those essential for life, contain at least one atom of carbon; combined with hydrogen, oxygen, nitrogen, sulfur, and phosphorus, carbon is the basis of every important biological compound.
What are the elements in period 2?
Period 2. Period 2 elements involve the 2s and 2p orbitals. They include the biologically most essential elements besides hydrogen: carbon, nitrogen, and oxygen. Lithium (Li) is the lightest metal and the least dense solid element. In its non-ionized state it is one of the most reactive elements, and so is only ever found naturally in compounds.
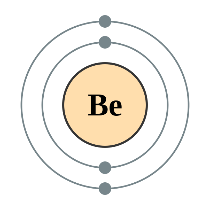
How does quantum mechanics explain periodic trends?
Modern quantum mechanics explains these periodic trends in properties in terms of electron shells. As atomic number increases, shells fill with electrons in approximately the order shown in the ordering rule diagram. The filling of each shell corresponds to a row in the table.
Which element is the fourth most abundant in the universe?
Carbon (C) is the fourth-most abundant element in the universe by mass after hydrogen, helium and oxygen and is the second-most abundant element in the human body by mass after oxygen, the third-most abundant by number of atoms.
Which element is the most reactive?
Fluorine (F) is the most reactive element in its non-ionized state, and so is never found that way in nature.
What is a period in chemistry?
Definition of period - Chemistry Dictionary. Chemistry Dictionary. Definition of Period. A period is the name given to a horizontal row of the periodic table. The periodic table has seven periods. Apart from period 1, every period begins with an alkali metal and ends with a noble gas. Period 1 contains only two elements: hydrogenand helium.
Which period contains only two elements?
Period 1 contains only two elements: hydrogenand helium. Periods 6 and 7 are the longest periods, each containing 32 elements. Period 6 includes the lanthanides running from lanthanumto lutetium. Period 7 includes the actinides running from lanthanumto oganesson).
Learn about this topic in these articles
The horizontal rows of the periodic table are called periods. Each period corresponds to the successive occupation of the orbitals in a valence shell of the atom, with the long periods corresponding to the occupation of the orbitals of a d subshell. Successive periods down the table correspond to successively…
basis of periodic table
The horizontal rows of the periodic table are called periods. Each period corresponds to the successive occupation of the orbitals in a valence shell of the atom, with the long periods corresponding to the occupation of the orbitals of a d subshell. Successive periods down the table correspond to successively…
What is the periodic table?
The periodic table is a tabular arrangement of the chemical elements by increasing atomic number which displays the elements so that one may see trends in their properties.
How are periodic tables arranged?
Key Takeaways: Periodic Table Definition 1 The periodic table is a tabular arrangement of chemical elements that is arranged by increasing atomic number and groups elements according to recurring properties. 2 The seven rows of the periodic table are called periods. The rows are arranged so that metals are on the left side of the table and nonmetals are on the right side. 3 The columns are called groups. Group contain elements with similar properties.
What are the elements that make up the majority of the periodic table?
The majority of chemical elements are metals. Metals tend to be shiny (metallic luster), hard, conductive, and capable of forming alloys. Nonmetals tend to be soft, colored, insulators, and capable of forming compounds with metals. Metalloids display properties intermediate between those of metals and nonmetals. Toward the right side of the periodic table, the metals transition into nonmetals. There is a rough staircase pattern—starting at boron and going through silicon, germanium, arsenic, antimony, tellurium, and polonium—that identified the metalloids. However, chemists increasingly categorize other elements as metalloids, including carbon, phosphorus, gallium, and others.
What are the rows on the periodic table called?
The seven rows of the periodic table are called periods . The rows are arranged so that metals are on the left side of the table and nonmetals are on the right side. The columns are called groups. Group contain elements with similar properties.
How many rows are there in the periodic table?
There are seven rows of the periodic table, which are called periods. Element atomic number increases moving from left to right across a period. Elements toward the left side of a period are metals, while those on the right side are nonmetals. Moving down a period on the table adds a new electron shell.
Where are the elements on the periodic table?
Metallic character is highest at the bottom lefthand corner of the periodic table, while the most nonmetallic elements are in the upper righthand corner.
What are the columns of elements called?
The columns of elements are called groups or families . Groups are numbered from 1 (the alkali metals) to 18 (the noble gases). Elements with a group share a valence electron configuration. Elements within a group display a pattern with respect atomic radius, electronegativity, and ionization energy.
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
Why is period 1 the shortest period?
Period 1 of the periodic table is given the name shortest period because there are only two elements in period 1.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
