See more
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the F block?
The f-block consists of two series lanthanides and actinides of the periodic table.
What is the modern periodic table?
The modern periodic table is the arrangement of all the known elements such that elements with similar characteristics are grouped. We know that the modern periodic table is made up of horizontal periods and vertical groups. In this article, we are going to study the similarities and periodicity of elements across the periodic table. We will study the different blocks: s-block, p-block, d-block, f-block and their characteristic properties, metals, metalloids, nonmetals, halogens, and noble gases.
How many metalloids are there?
There are 7 metalloids i.e. Boron, Silicon, germanium, arsenic, antimony, tellurium and polonium.
What type of electron enters in a D block?
In d-block elements, a valence electron enters in d-orbital.
What is the electronic configuration of halogens?
The general electronic configuration of halogens is ns2np5.
Which elements of Group 1 and Group 2 belong to the S block?
The elements of Group 1 ( alkali metals) and Group 2 ( alkaline earth metals) which have ns1 and ns2 outermost electronic configuration belong to the s-Block Elements.
What are the shiny elements that form naturally below the surface of the Earth?
The lustrous or shiny elements that form naturally below the surface of the Earth are called Metals. These are elements having positive ions.
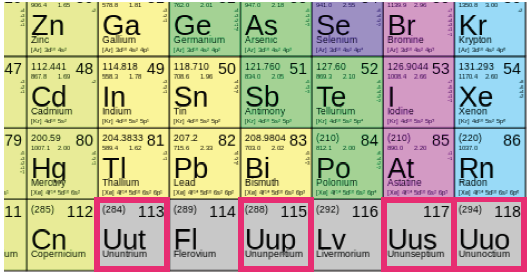
Where are the F block elements in the periodic table?
The position of F Block Elements in the Periodic Table: F block elements are placed separately at the bottom of the periodic table. They are a subset of 6th and 7th periods.
What are F Block Elements?
Elements whose f orbital getting filled up by electrons are called f block elements. These elements have electrons, (1 to 14) in the f orbital, (0 to 1) in the d orbital of the penultimate energy level and in the outermost’s orbital.
Which element is involved in the filling of 4F orbitals?
Lanthanoids are involved in the filling of 4f- orbitals whereas actinoids are involved in the filling of 5f-orbitals. The binding energy of 4f electrons is comparatively less than that of 5f-electrons. The shielding effect of 5f-electrons is less effective as compared to that of 4f-electrons.
What are the first series of elements?
The first series of elements are called lanthanides and include elements with atomic numbers beginning from 57 and ending at 71. These elements are non-radioactive (except for promethium, which is radioactive).
How many series are in the F block?
There are two series in the f block corresponding to the filling up of 4f and 5f orbitals. The elements are 4f series of Ce to Lu and 5f series of Th to Lw. There are 14 elements filling up the ‘f’ orbital in each series.
Which row of the periodic table is the inner transition metal?
These block of elements are often referred to as inner transition metals because they provide a transition in the 6th and 7th row of the periodic table which separates the s block and the d block elements.
Is 5F a shielding effect?
The shielding effect of 5f-electrons is less effective as compared to that of 4f-electrons. The paramagnetic properties of lanthanoids can be easily explained but this explanation is difficult in the case of actinoids.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Do elements of the same group have the same number of valence electrons?
In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
Why is period 1 the shortest period?
Period 1 of the periodic table is given the name shortest period because there are only two elements in period 1.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
Do elements in the same period have the same number of energy shells?
The elements in same Periods have the same number of energy shells (or energy orbits).
