
Is Si a nonmetal or metal?
Silicon is not a metal, it is a non-metal,Silicon is a non-metallic chemical element. Its chemical symbol is Si, formerly known as silicon,The atomic number is 14, the relative atomic mass is 28.0855; Crystalline silicon looks like black hard coal, not like metal.
What is period 3 on the periodic table?
What is Period 3 on the periodic table? The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block.
What is element 59 on the periodic table?
She has taught science courses at the high school, college, and graduate levels. Praseodymium is element 59 on the periodic table with the element symbol Pr. It's one of the rare earth metals or lanthanides. Here is a collection of interesting facts about praseodymium, including its history, properties, uses, and sources.
What element has the longest name?
mycro writes "A new article on Wikipedia shows the longest chemical name, reaching 64,060 letters. Methionylalanylthreonyl...leucine is a chemical name for enaptin, a nuclear envelope protein found in human myocytes and synapses, which is made up of 8,797 amino acids. It is involved in the maintenance of nuclear organization and structural integrity, tethering the cell nucleus to the cytoskeleton by interacting with the nuclear envelope and with F-actin in the cytoplasm."
See more

Is silicon a metal?
Is silicon a metal? No, silicon is classified as a metalloid because some of its properties resemble the properties of metals and some of its properties resemble those of nonmetals.
What is SI commonly known as?
The International System of Units (SI), commonly known as the metric system, is the international standard for measurement. The International Treaty of the Meter was signed in Paris on May 20, 1875 by seventeen countries, including the United States and is now celebrated around the globe as World Metrology Day .
What are 5 uses of silicon?
Uses of SiliconThe element is a major constituent in ceramics and bricks.Being a semiconductor, the element is put into use for making transistors.Silicon is widely used in computer chips and solar cells.It is a vital component of Portland cement.Silicon is used in the production of fire bricks.More items...•
What type of metal is Si?
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it.
What is the SI unit of time?
The secondThe second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the cesium frequency ΔνCs, the unperturbed ground-state hyperfine transition frequency of the cesium 133 atom, to be 9 192 631 770 when expressed in the unit Hz, which is equal to s-1.
What is silicon metal used for?
High-purity silicon metal is used by many industries. In the chemical industry it is used for producing silicon compounds as well as silicon wafers used in photovoltaic solar cells and electronic semiconductors. And aluminum manufacturers use it to improve the already useful properties of aluminum.
Why is silicon so important?
Silicon is one of the most useful elements to mankind. Most is used to make alloys including aluminium-silicon and ferro-silicon (iron-silicon). These are used to make dynamo and transformer plates, engine blocks, cylinder heads and machine tools and to deoxidise steel. Silicon is also used to make silicones.
Why is silicon important to life?
For example, silicon has been suggested to exhibit roles in the structural integrity of nails, hair, and skin, overall collagen synthesis, bone mineralization, and bone health and reduced metal accumulation in Alzheimer's disease, immune system health, and reduction of the risk for atherosclerosis.
What are 3 interesting facts about silicon?
Share This:1) Silicon gets its name from the Latin “silex,” meaning flint or hard stone. ... 2) Contrary to what some may think, silicon and silicone are quite different. ... 3) Pure silicon has the same crystal structure as diamond, which is made of carbon – the element that sits above silicon in the periodic table.More items...•
What's silicon made of?
Silicone itself is made up of carbon, hydrogen, oxygen and silicon. Note that the ingredient contained within silicone is spelt differently. The ingredient silicon comes from silica which is derived from sand.
Is silicon a metal or nonmetal Why?
Silicon is neither metal nor non-metal; it's a metalloid, an element that falls somewhere between the two. The category of metalloid is something of a gray area, with no firm definition of what fits the bill, but metalloids generally have properties of both metals and non-metals.
Is silicon hard to break?
Compared with pure metals and ionic salts, covalent solids such as silicon are hard and brittle because dislocations do not move in them except at high temperatures.
What does SI stand for in physics?
The International System of UnitsThe International System of Units, universally abbreviated SI (from the French Le Système International d'Unités), is the modern metric system of measurement.
What does SI stand for in math?
The International System of Metric Units S.I. is an abbreviation of Système Internationale or International System: our metric system of measurements.
What does SI mean in electricity?
the International System of UnitsSI is the International System of Units—in French, Système International d'Unités. It is the modern form of the metric system and is the most widely used system of measurement. The system was published in 1960 as the result of discussions that started in 1948. SI is based on the metre-kilogram-second system (MKS).
What does the French word SI mean in English?
“Yes”“Si” means “Yes” after a negative question or affirmation. “Si” also means “So much”, like “tellement.” “Si” is a musical note in French music.
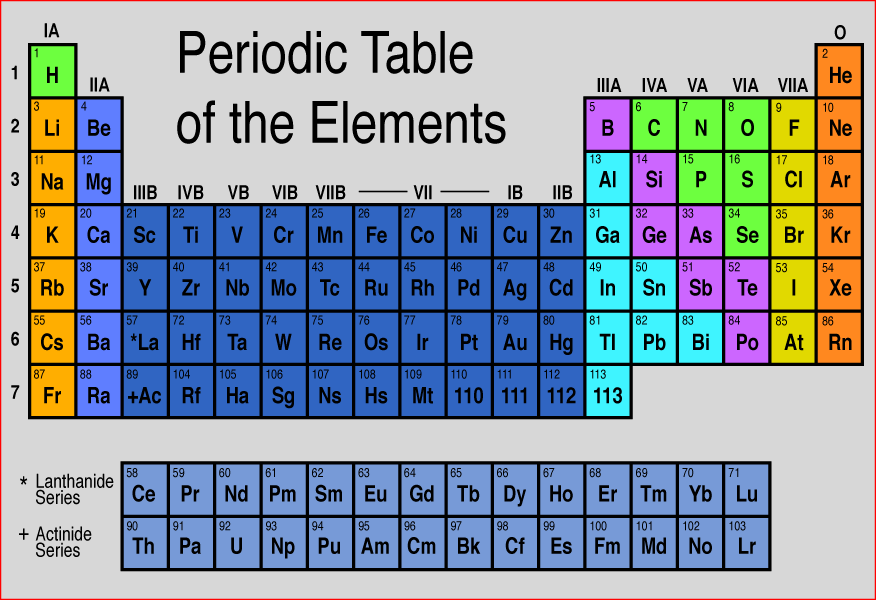
Silicon in Periodic table
Silicon element is in group 14 and period 3 of the Periodic table. Silicon is the p-block element and it belongs to carbon group.
Properties of Silicon
The physical and chemical properties of silicon elements are listed below.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
What is the vertical column in the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.
Which element is the element of life?
Well, it is often said that elements close to each other in the periodic table share similar properties and so, seduced by the age-old red herring that "carbon is the element of life", the writers selected the element below it, silicon.
What is silicon oil used for?
These are used to make dynamo and transformer plates, engine blocks, cylinder heads and machine tools and to deoxidise steel. Silicon is also used to make silicones. These are silicon-oxygen polymers with methyl groups attached. Silicone oil is a lubricant and is added to some cosmetics and hair conditioners.
What is the second most abundant element on Earth?
Silicon makes up 27.7% of the Earth’s crust by mass and is the second most abundant element (oxygen is the first). It does not occur uncombined in nature but occurs chiefly as the oxide (silica) and as silicates. The oxide includes sand, quartz, rock crystal, amethyst, agate, flint and opal.
How many oxygen atoms are in silicate rocks?
Silicate rocks - those in which silicon is surrounded tetrahedrally by four oxygen atoms - exist in an astonishing variety, the differences being determined by how the tetrahedra building blocks link together, and what other elements are present to complete the picture. When the tetrahedra link one to the next, one gets a mad tangle of chains looking like an enormous pot of spaghetti - the sorts of structures one gets in ordinary glass.
What is the most useful element?
The element, when ultrapure, is a solid with a blue-grey metallic sheen. Uses. Silicon is one of the most useful elements to mankind. Most is used to make alloys including aluminium-silicon and ferro-silicon (iron-silicon).
How are elements organized into blocks?
Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The number of protons in an atom.
What is silicon in the periodic table?
Overview of silicon, including mining and processing. Silicon (Si), a nonmetallic chemical element in the carbon family (Group 14 [IVa] of the periodic table). Silicon makes up 27.7 percent of Earth ’s crust; it is the second most abundant element in the crust, being surpassed only by oxygen. Chemical properties of the element silicon.
Which elements exceed silicon?
Only hydrogen, helium, oxygen, neon, nitrogen, and carbon exceed silicon in cosmic abundance. Silicon is believed to be a cosmic product of alpha-particle absorption, at a temperature of about 10 9 K, by the nuclei of carbon-12, oxygen-16, and neon-20.
How does silica cycle?
It cycles through the marine environment, entering primarily through riverine runoff. Silica is removed from the ocean by organisms such as diatoms and radiolarians that use an amorphous form of silica in their cell walls. After they die, their skeletons settle through the water column, and the silica redissolves. A small number reach the ocean floor, where they either remain, forming a silaceous ooze, or dissolve and are returned to the photic zone by upwelling.
Why is silicon a base element?
Because silicon forms chains similar to those formed by carbon, silicon has been studied as a possible base element for silicon organisms. The limited number of silicon atoms that can catenate, however, greatly reduces the number and variety of silicon compounds compared with those of carbon.
Where does the name silicon come from?
The name silicon derives from the Latin silex or silicis, meaning “flint” or “hard stone.”. Amorphous elemental silicon was first isolated and described as an element in 1824 by Jöns Jacob Berzelius, a Swedish chemist. Impure silicon had already been obtained in 1811. Crystalline elemental silicon was not prepared until 1854, ...
What percent of the Earth's crust is silicon?
Silicon makes up 27.7 percent of Earth ’s crust; it is the second most abundant element in the crust, being surpassed only by oxygen. Chemical properties of the element silicon. The periodic table is made up of 118 elements.
How many electron volts does the nucleus of iron have?
Compared with the maximum of about 8.7 million electron volts for the nucleus of iron, almost twice as massive as that of silicon, this figure indicates the relative stability of the silicon nucleus. Earth's crust composition. The mineral composition of Earth's crust. Encyclopædia Britannica, Inc.
How many isotopes of silicon are there?
Isotopes of Silicon. Silicon has three stable isotopes; Si-28, Si-29 and Si-30. Of these three naturally occurring isotopes Si-28 is the most abundant as it is produced in stars as well as during nuclear fusion reaction. The remaining two isotopes of silicon form only 7% of the naturally occurring silicon.
What are the physical characteristics of silicon?
Physical Characteristics. Silicon is a brittle and hard crystalline solid. It has blue-grey metallic lustre. Silicon, in comparison with neighbouring elements in the periodic table, is unreactive. The symbol for silicon is Si with atomic number 14. It has a very high melting and boiling point.
What are silicon minerals used for?
Silicon minerals are used as structural compounds for instance as clays, silica sand, building mortar, stucco and building stones. Silicon minerals are used in making concrete. Silica is used to make fire brick (refractory brick) which is used in lining of furnace.
What is the second most abundant element in the Earth's crust?
Silicon. Silicon is the second most abundant element in earth’s crust. It was discovered in 1823 by Jöns Jacob Berzelius. Silicon has tremendous uses including manufacturing of ceramic, glass, synthetic polymers and is an essential part of integrated circuits.
How is silicon formed?
It is the seventh most abundant element in the universe. Silicon is formed through the oxygen-burning process in stars. Silicon reacts with oxygen to make silicon dioxide or silicates. Silicate minerals make up over 90% of earth’s crust. Silicon is rarely found in pure form.
What is silica used for?
Silica is used in making optical fibre which has vast uses in telecommunications and computer networking.
What is the atomic number of antimony?
Antimony is a chemical element with symbol Sb and atomic number 51. A lustrous gray…
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
What is periodic table?
Periodic table showing the relative sizes of the elements based on atomic radius data. Todd Helmenstine
What happens to the atoms as you move across an element period?
As you move across an element period (row), the overall size of atoms decreases slightly. Even though atoms further to the right have more protons, neutrons, and electrons, the outer electron shell is the same. The increased number of protons exerts a stronger positive charge, pulling the electrons in toward the nucleus.
Why does the atomic radius increase as you move down an element group?
As you move down an element group (column), the size of atoms increases. This is because each atom further down the column has more protons and neutrons and also gains an additional electron energy shell.
Occurrence
Physical Characteristics
- Silicon is a brittle and hard crystalline solid. It has blue-grey metallic lustre. Silicon, in comparison with neighbouring elements in the periodic table, is unreactive. The symbol for silicon is Si with atomic number 14. It has a very high melting and boiling point. At standard conditions silicon also makes a giant covalent structure like other g...
Chemical Characteristics
- At room temperature, pure silicon acts as an insulator. Silicon is a semiconductor at standard temperature and pressure. Silicon is inert in crystalline form at low temperatures. Its conductivity increases with high temperature. Silicon readily reacts with oxygen . It reacts with air above 900-degree centigrade. Melted silicon becomes very reactive and has to be stored in unreactive, refr…
Significance and Uses
- Silicon minerals are used as structural compounds for instance as clays, silica sand, building mortar, stucco and building stones.
- Silicon minerals are used in making concrete.
- Silica is used to make fire brick (refractory brick) which is used in lining of furnace.
- It is used in making whiteware ceramics such as soda lime glass and porcelain.
Health Effects
- Silicon is slightly hazardous. If crystalline silica is inhaled, it may lead to lung disease such as asthma or inflammation in upper lobes of lungs. Exposure of elemental silicon can cause eye or skin irritation.
Isotopes of Silicon
- Silicon has three stable isotopes; Si-28, Si-29 and Si-30. Of these three naturally occurring isotopes Si-28 is the most abundant as it is produced in stars as well as during nuclear fusion reaction. The remaining two isotopes of silicon form only 7% of the naturally occurring silicon. So far twenty radioisotopes of silicon have been characterized. Most of these radioisotopes have h…