
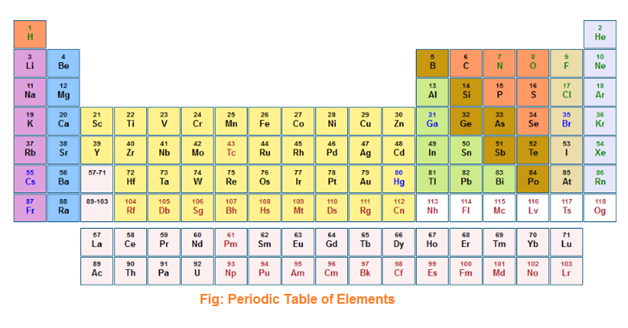
What are the first 20 elements?
These are the first 20 elements, listed in order: H - Hydrogen He - Helium Li - Lithium Be - Beryllium B - Boron C - Carbon N - Nitrogen O - Oxygen F - Fluorine Ne - Neon Na - Sodium Mg - Magnesium Al - Aluminum Si - Silicon P - Phosphorus S - Sulfur Cl - Chlorine Ar - Argon K - Potassium Ca - ...
How do you find elements in the periodic table?
How do you find elements in the periodic table? To find the number of electrons an element has, locate it on the periodic table of elements, find the atomic number, and note the number of protons; because atoms are naturally electrically neutral, the protons and electrons are usually equal. Look at the oxidation number for further information.
What determines the order for elements on the periodic table?
The periodic table is the table that indexes all chemical elements. It is arranged in order of atomic size (Hydrogen, then Helium, then Lithium, etc.) which is determined by the number of protons in the element. The table is subdivided into periods (rows) and groups (columns) of elements that share common characteristics, hence the name "periodic".
What is the first twenty element?
What are the metal (s) and non-metals in the first 20 elements? Lithium, Beryllium, Sodium, Magnesium, Aluminium, Potassium, and Calcium are metals in the first twenty elements. Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon are the non-metals in the first twenty elements.
Who created the periodic table?
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements, like he was playing a game, while traveling by train.
Can periodic table games be used for grade?
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
Does the Modern Periodic Table Change? If So, How and Who Does That?
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack).
What order are the first 20 elements in the periodic table?
This is also the number of protons in each atom. These are the first 20 elements, listed in order: H - Hydrogen.
What does the number of an element mean?
The number of the element is its atomic number, which is the number of protons in each atom of that element. The element symbol is a one- or two-letter abbreviation of the element's name. Sometimes it refers to an old name. (For instance, K is for kalium.) The element name can tell you something about its properties.
How to tell how many neutrons an atom has?
This is indicated using numbers (superscripts, subscripts, or following the symbol) to give the total number of protons and neutrons.
What are the names of the elements that end with gen?
Elements that have names ending with - ine belong to a group of elements called halogens. Halogens are extremely reactive and readily form compounds. Element names ending with - on are noble gases, which are inert or nonreactive gases ...
What does the superscript after the element symbol mean?
Ions indicated using a superscript after the element symbol that states whether the charge on the atom is positive (more protons) or negative (more electrons) and the quantity of the charge. For example, Ca 2+ is the symbol for a calcium ion that has a positive 2 charge.
What are the elements that make up a substance?
Chemical Elements. To be an element, a substance has to at least have protons, since these particles define the type of element. Elements consist of atoms , which contain a nucleus of protons and neutrons surrounded by a cloud or shell of electrons.
How many protons does carbon 14 have?
For example, carbon-14 has 14 protons and neutrons. Since you know all atoms of carbon have 6 protons, the number of neutrons is 14 - 6 = 8. Ions are atoms that have different numbers of protons and electrons.
How many elements are in the periodic table?
Based on an earlier (1882) model of T. Bayley, J. Thomsen in 1895 devised a new table. This was interpreted in terms of the electronic structure of atoms by Niels Bohr in 1922. In this table there are periods of increasing length between the noble gases; the table thus contains a period of 2 elements, two of 8 elements, two of 18 elements, one of 32 elements, and an incomplete period. The elements in each period may be connected by tie lines with one or more elements in the following period. The principal disadvantage of this table is the large space required by the period of 32 elements and the difficulty of tracing a sequence of closely similar elements. A useful compromise is to compress the period of 32 elements into 18 spaces by listing the 14 lanthanoids (also called lanthanides) and the 14 actinoids (also called actinides) in a special double row below the other periods.
Who suggested that the atomic number be identified with the ordinal number of the element in the periodic system?
In 1911 A. van den Broek suggested that this quantity, the atomic number, might be identified with the ordinal number of the element in the periodic system (following the lead of Newlands, it had become customary to number the elements according to their position in the table).
What is the long period form of the periodic system?
Long-period form of periodic system of elements. Encyclopædia Britannica, Inc. With the discovery of the noble gases helium, neon, argon, krypton, radon, and xenon by Lord Rayleigh (John William Strutt) and Sir William Ramsay in 1894 and the following years, Mendeleyev and others proposed that a new “zero” group to accommodate them be added to ...
What elements did Mendeleyev predict?
Mendeleyev was also able to predict the existence, and many of the properties, of the then undiscovered elements eka-boron, eka-aluminum, and eka-silicon, now identified with the elements scandium, gallium, and germanium, respectively. Similarly, after the discovery of helium and argon, the periodic law permitted the prediction of the existence ...
How does the atomic weight of an element show its position in the periodic system?
That the exact atomic weight of an element is of small significance for its position in the periodic system is shown by the existence of isotopes of every element —atoms with the same atomic number but different atomic weights. The chemical properties of the isotopes of an element are essentially the same, and all the isotopes of an element occupy the same place in the periodic system in spite of their differences in atomic weight.
Which elements were put in positions out of the order of atomic weights?
In the pairs argon and potassium, cobalt and nickel, and tellurium and iodine, for example, the first element had the greater atomic weight but the earlier position in the periodic system. The solution to this difficulty was found only when the structure of the atom was better understood.
How many spaces are there in a period of 32?
A useful compromise is to compress the period of 32 elements into 18 spaces by listing the 14 lanthanoids (also called lanthanides) and the 14 actinoids (also called actinides) in a special double row below the other periods.
