Which halogen has the greatest reactivity?
Which halogen has the highest reactivity? Fluorine is the most reactive of the halogens and, in fact, of all elements, and it has certain other properties that set it apart from the other halogens. Chlorine is the best known of the halogen elements.
What halogen is most likely to react?
What Halogen is most likely to react? Fluorine is the most reactive of the halogens and, in fact, of all elements, and it has certain other properties that set it apart from the other halogens. Chlorine is the best known of the halogen elements.
Why are halogens the most reactive of the nonmetal elements?
The halogen group of elements is the most reactive of the nonmetals. It is also the most reactive group of all chemical elements. Fluorine is the most reactive element in this group. Halogens are highly reactive because they are all electronegative. Which nonmetal in Period 2 is most reactive?
Why is astatine the least reactive halogen?
This is because chlorine could displace bromine and iodine, bromine could only displace iodine, but iodine could not displace chlorine or bromine. In respect to this, which halogens are most reactive? As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. All halogens form Group 1 salts with similar properties.

Which halogen is most reactive?
FluorineFluorine is the most reactive element of all in Group 7. You can see the trend in reactivity if you react the halogens with iron wool. Reacts with almost anything instantly. Very few scientists handle fluorine because it is so dangerous.
What is the least reactive halogen on the periodic table?
The bonds in these diatomic molecules are non-polar covalent single bonds. However, halogens readily combine with most elements and are never seen uncombined in nature. As a general rule, fluorine is the most reactive halogen and astatine is the least reactive.
Which one of the following is most reactive cl2 F2 br2 i2?
F2 is most reactive halogen.
Which is more reactive F or Br?
Among the halogens, fluorine, chlorine, bromine, and iodine, fluorine is the most reactive one.
Why is fluorine the most reactive halogen?
Fluorine has the shortest atomic size of all the halogens. As a result, the nuclear attraction on the furthest electrons is greatest. Among the halogens, fluorine is the most reactive.
Why halogens are most reactive elements?
Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more reactive than other non-metal groups.
Which among the following is the most reactive 1 Cl2 2 br2 3 i2 4 ICl?
Hence, ICl is most reactive.
Which is more reactive Cl2 or br2?
Why: Chlorine is more reactive the bromine because chlorine radical is less stable then bromine radical.
Which of the following is most reactive a Cl2 B i2 C br2 D ICl?
The iodine and chlorine have differences in their electronegativity values then the compound ICl is polar in nature. - Means $ICl$ is highly reactive when compared to the remaining molecules given in the options. So, the correct answer is “Option D”.
Which is more reactive FL or Cl?
1] fluorine is more reactive than chlorine .
Which of the following is the most reactive halogen A F B Cl C Br d I?
fluorineThus, the most reactive halogen is fluorine.
Why Cl is more reactive than cl2?
While when same halogens form X2(like cl2) molecules and they form covalent bonds which are stronger than interhalogen compound and weak bond obviously is more reactive than the stronger bond and that's why ICI is more reactive than cl2.
What is least reactive element on the periodic table?
Noble gasesNoble gases are the least reactive of all elements. That's because they have eight valence electrons, which fill their outer energy level. This is the most stable arrangement of electrons, so noble gases rarely react with other elements and form compounds.
What is the least reactive group of elements on the periodic table?
Noble gases cannot gain or lose valence electrons by interacting with other elements, making them most stable or least reactive.
Which element in Group 7 is least reactive?
astatineAs you go down Group 7, the reactivity of halogens decreases so fluorine is the most reactive halogen and astatine is the least reactive halogen.
How do you find the least reactive element on the periodic table?
The group-18 elements are least reactive and are referred to as inert or noble gases as they have a very stable electronic configuration which is why they do not react with other elements to lose or share their electrons.
What are the physical properties of the halogens?
The physical properties of halogens are as follows. The halogens may exist as solid, liquid, or gas at room temperatures. They dissolve in water...
Where are the halogens found?
They are found in group 17 of the Periodic Table, on the extreme right, close to the noble gases. Their valence electron configuration is the reaso...
Why are Group 7 elements called halogens?
They are called halogens as they form salts with metals. The term "halogen" is derived from the Greek language and means salt producers.
Why are halogens reactive?
To the question, " are halogens reactive", the answer is yes and the reason lies in their electron configuration. Atoms of all the halogens have seven valence electrons. The halogen atoms are very close to a stable electron configuration. This explains why halogens are reactive.
Where Are Halogens on the Periodic Table?
It places elements with similar chemical properties in groups. The halogens are located in group 17 on the periodic table.
What are Halogens?
Halogens is the collective name given to a group of non-metallic elements. The name "halogens" owes its origin to the Greek language. "Halogens" means "salt formers". Halogens can be defined as elements that form salts when they react with metals. There are six halogens- fluorine, chlorine, bromine, iodine, astatine, and tennessine. Tennessine is the latest addition to the halogen family and is named after the state of Tennessee, US; but it is still not considered a true halogen because it is a synthetic element.
Why are halogens always found in the combined form?
The reactive nature of halogens is the reason why they are always found in the combined form.
What is the boiling point of iodine?
Boiling points: The boiling points of the halogens show a regular gradation. The boiling points from fluorine to iodine are 85.01K, 239.18K, 331.93K, and 457.5K. The boiling point of astatine is not known. This trend in the halogens' property is due to the van der Waal's forces existing in the halogen molecules. These forces are stronger when the atoms have a large number of electrons. More energy is required to overcome these forces. This results in a graded increase in boiling points.
Why are halogens found only as compounds?
Halogens are never found in nature as elements; they are found only as compounds because of their reactive nature.
How does reactivity affect the group?
Reactivity: Reactivity can be explained as to how easily an element forms compounds with other elements. Halogens' reactivity decreases down the group. The halogens' electron configuration is {eq}ns^ {2}np^ {5} {/eq}. The halogen atoms are just one electron short of completing their octet. So, the halogens accept an electron to fill their shells. As the halogens' atom size increases, it is difficult for the large atoms to take in the extra electron. The attractive force the nucleus exerts on the incoming electron diminishes down the group. So reactivity of halogens decreases on moving down. This trend is shown as: {eq}F > Cl > Br > I > At {/eq}.
Which group of elements are less reactive?
The non-metal elements in Group 7 - known as the halogens - get less reactive as you go down the group. This is the opposite trend to that seen in the alkali metals in Group 1 of the periodic table.
What are the elements in Group 7?
The Group 7 elements are known as the halogens. They are reactive non-metals and are always found in compounds with other elements. Chlorine, bromine and iodine are all halogens. Part of. Chemistry (Single Science)
What are the two substances that react with iron wool?
You can see the trend in reactivity if you react the halogens with iron wool. Halogen. Reaction with iron wool. Fluorine. Reacts with almost anything instantly. Very few scientists handle fluorine because it is so dangerous. Chlorine. Reacts with heated iron wool very quickly. Bromine.
Where are halogens found in the periodic table?
The halogen elements are located in group VIIA of the periodic table, which is the second-to-last column of the chart. This is a list of elements that belong to the halogen group and the properties that they share in common:
What color is halogen?
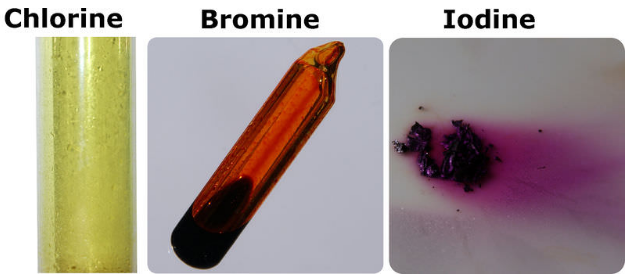
The halogens are colorful, even as gases. Fluorine is the palest element, but even as a gas it has a distinct yellow color.
What are halogens used for?
Their high reactivity also makes these elements important components of some types of bleach. Halogens are used in incandescent lamps to make them glow at a higher temperature and with a white color. The halogen elements are important drug components, as they aid drug penetration into tissues.
Why do halogens need more electrons?
These atoms need one more electron in order to have a stable octet. Halogens are highly electronegative, with high electron affinities. The melting and boiling points of the halogens increase as you increase atomic number (as you move down the periodic table).
How many electrons are in a halogen atom?
They are highly reactive nonmetals. Atoms of belonging to the halogen group have 7 electrons in their outermost (valence) shell.
What is the element Br?
Bromine is element 35 with symbol Br. It is a liquid at room temperature and pressure.
Is chlorine a halogen?
Fluorine, chlorine, bromine, iodine, and astatine definitely are halogens. Element 117, which has the placeholder name of ununseptium, might have some properties in common with the other elements. Even though it is in the same column or group of the periodic table with the other halogens, most scientists believe element 117 will behave more like ...
How many halogens are there on the periodic table?
There are 5 known halogens on the Periodic table.
Where are halogens located on the periodic table?
Halogens are located in the 17th group of Periodic table (exactly to the left of Noble gases). Well, I hope you have got the exact answer for your question “Where are the halogens located on the periodic table?” from this above image.
What are the chemical properties of halogens?
Chemical properties of halogens. #1 Halogens or group 17 elements are highly reactive nonmetals. #2 Halogens have 7 electrons in their outermost orbit and they easily gain one electron to form a stable octet. #3 All the halogen elements are poisonous. #4 All the halogens molecules are diatomic molecules.
What does halogen mean?
The word halogen has derived from two Greek words . So halogens means salt producing. In other words, these halogen elements form salts when they react with metals. Here chlorine (Cl) is a halogen, and when it reacts with metals (sodium, Na), it will form a salt (NaCl). There are 5 known halogens on the Periodic table.
Which element is the most reactive?
Now see, the halogens are the most reactive elements, but we know that as we move down the group, the electronegativity decreases (means the tendency to attract the electron pair decreases down the group).
Which element has the highest tendency to react with any other element to form a compound?
And because of this, fluorine has a highest tendency to react with any other element to form a compound. Even fluorine reacts with noble gas like xenon, and forms compounds like XeF4 (Xenon tetrafluoride) and XeF6 (Xenon hexafluoride) Thus, fluorine element of group 17 is a highly reactive halogen on the Periodic table.
How long does Astatine-210 last?
And it has a very short half life. The longest half life of a stable isotope Astatine-210 is 8.1 hours and of Astatine-211 is 7.2 hours.