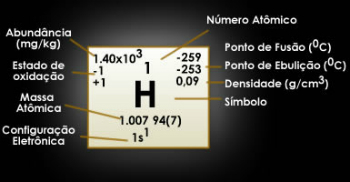
What group and period does hydrogen belong to?
Hydrogen is a very special element of the periodic table and doesn't belong to any family. While hydrogen sits in Group I, it is NOT an alkali metal. Further detail about this can be seen here. Also asked, what group and period is hydrogen in? Similarly, is hydrogen a member of Group 1? Group 1: Hydrogen and the Alkali Metals.
What period is strontium in?
Strontium element is in group 2 and period 5 of the Periodic table. Strontium is the s-block element and it belongs to alkaline earth metals group.
What period is argon in?
Argon element is in group 18 and period 3 of the Periodic table. Argon is the p-block element and it belongs to Noble gases group.
What are some interesting facts about hydrogen?
Fun Facts About Hydrogen!
- About 10 percent of the weight of living organisms is hydrogen – mainly in water, proteins and fats.
- Liquid hydrogen has the lowest density of any liquid.
- Solid, crystalline hydrogen has the lowest density of any crystalline solid.
- Hydrogen is the only element that can exist without neutrons. ...
See more

Is hydrogen a period 1?
The first period has less elements than any other periods in the periodic table. There are only two elements in the first period: hydrogen and helium. We can explain why there is less elements in the first row in modern theories of atomic structure.
Does hydrogen have a period?
Hydrogen (H) is the most abundant of the chemical elements, constituting roughly 75% of the universe's elemental mass. Ionized hydrogen is just a proton. Stars in the main sequence are mainly composed of hydrogen in its plasma state....Period 1.Group118Atomic # Name1 H2 He
What is Period 1 on the periodic table?
0:292:53Periods & Groups In The Periodic Table | Properties of Matter - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach new period row represents a new shell elements in the first period have one shell and as we goMoreEach new period row represents a new shell elements in the first period have one shell and as we go down the shells.
What is group of hydrogen?
Hydrogen is placed in group - I as it shows similarities in properties with alkali metals (Li, Na, K, etc.). Identify such similar properties between Hydrogen (H) and Alkali metals (M). Q. Mendeleev placed hydrogen in the 1st group along with alkali metals.
What element is in period 2?
0:0221:31Period 2 element ⚛️ Periodic Table of Elements Series ⚛️ - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe second period contains the elements lithium beryllium boron carbon nitrogen oxygen fluorine. AndMoreThe second period contains the elements lithium beryllium boron carbon nitrogen oxygen fluorine. And neon.
Which element is in period 3 and Group 2?
magnesiumHence the name of the element is magnesium.
What are the 7 periods in periodic table?
Period 7 elementHydrogenHeliumLithiumBerylliumNeonSodiumMagnesiumArgonPotassiumCalciumKryptonRubidiumStrontiumXenon2 more rows
What element is Group 2 Period 2?
Group 2A (or IIA) of the periodic table are the alkaline earth metals: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)....Group 2A — The Alkaline Earth Metals.21ALi2ABe4AC12 more columns
What element is in group 1 period 3?
Metal present in group 1 and period 3 is sodium. The symbol for sodim is Na. Its electronic configuration is 2,8,1. As it has 1 valence electron it belongs to group 1 and it has 3 electronic shells filled hence belongs to period 3.
Why is hydrogen in the first group?
Hydrogen is placed in group one because it has certain properties of group I elements. Hydrogen, like other alkali metals, is a powerful reducing agent. Hydrogen has an outer electronic configuration 1 s 1 that is comparable with those of group I elements.
Is hydrogen a nonmetal?
Properties of Hydrogen Hydrogen is a nonmetal and is placed above group in the periodic table because it has ns1 electron configuration like the alkali metals. However, it varies greatly from the alkali metals as it forms cations (H+) more reluctantly than the other alkali metals.
Why hydrogen can be placed in group 1 or 17?
The reason why the hydrogen can be placed in both group 1 and group 17 is that it resembles Alkali metals in some of its properties like it can easily form cations so, it can be placed in Group 1 of periodic table but it also resembles Halogens in its properties like it forms H2 which is true property of halogen that ...
Is hydrogen H or H2?
Hydrogen has a molar mass of 1 and it's molecular formula is H2. Hydrogen, H, is the lightest element with the atomic number 1. It is a colorless, odorless, tasteless, and highly flammable gas with the molecular formula H2.
What is the notation of hydrogen?
symbol HHydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element.
Is H2 an element?
H2 which is hydrogen is not a compound but an element as it is composed of a single element.
Is hydrogen a diatomic?
As a result, its tendency to exist in the monoatomic form is rather low. Instead, hydrogen forms a covalent bond with another hydrogen atom and exists as a diatomic (H2) molecule.
1. What is Special About Hydrogen?
Hydrogen is defined as the most abundant and simplest element in the universe. It consists of a single proton and electron. Liquid hydrogen can be...
2. Explain whether Hydrogen is gas or metal?
Hydrogen is the compound, which is most often classified as a nonmetal because it has several properties of nonmetal. For example, at room temperat...
3. Where is the Hydrogen molecule commonly used?
Hydrogen is used in the glass industry to make flat glass sheets as a protective atmosphere. It can also be used as a flushing gas in the electroni...
4. Give Two Chemical Properties of Oxygen?
Two element atoms bind to produce di-oxygen at the Standard Temperature and Pressure (STP), an odourless, colourless, tasteless diatomic gas having...
5. What are the Isotopes of Hydrogen and how do they differ from Hydrogen?
There are in total three isotopes of hydrogen which can be provided as follows:HydrogenDeuteriumTritiumThese are called to be isotopes as they have...
6. Can Hydrogen react with all elements in the periodic table or is it limited to only specific elem...
Hydrogen is a nonmetal that can easily react with a lot of elements and it also can form covalent bonds with a lot of elements. The reaction with v...
7. How is Hydrogen similar to Alkali Metals?
The similarities of hydrogen to metals can be provided as follows:Electronic configuration: Like all the other elements Hydrogen will consist of on...
8. How is Hydrogen different from a halogen?
The differences between halogens can be provided as follows:Structure of atom:Hydrogen will consist of only one electron in its outer shell while t...
9. What are some of the applications of Hydrogen in Chemistry?
The majority of hydrogen that has been industrially produced is used in Haber’s process to manufacture ammonia. It is also widely used in the produ...
Where is hydrogen found in the universe?
Natural abundance. Hydrogen is easily the most abundant element in the universe. It is found in the sun and most of the stars, and the planet Jupiter is composed mostly of hydrogen. On Earth, hydrogen is found in the greatest quantities as water.
How is hydrogen produced?
Any hydrogen that does enter the atmosphere quickly escapes the Earth’s gravity into outer space. Most hydrogen is produced by heating natural gas with steam to form syngas (a mixture of hydrogen and carbon monoxide). The syngas is separated to give hydrogen. Hydrogen can also be produced by the electrolysis of water.
What is hydrogen used for?
In the chemical industry it is used to make ammonia for agricultural fertiliser (the Haber process) and cyclohexane and methanol, which are intermediates in the production of plastics and pharmaceuticals. It is also used to remove sulfur from fuels during the oil-refining process.
What is the oxidation state of an atom?
The oxidation state of an atom is a measure of the degree of oxidation of an atom. It is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an oxidation state of 0. The sum of the oxidation states within a compound or ion must equal the overall charge.
What is a vertical column in the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table.
Is hydrogen gas a clean fuel?
Some see hydrogen gas as the clean fuel of the future – generated from water and returning to water when it is oxidised. Hydrogen-powered fuel cells are increasingly being seen as ‘pollution-free’ sources of energy and are now being used in some buses and cars. Hydrogen also has many other uses.
Who discovered that hydrogen is flammable?
In the early 1500s the alchemist Paracelsus noted that the bubbles given off when iron filings were added to sulfuric acid were flammable. In 1671 Robert Boyle made the same observation. Neither followed up their discovery of hydrogen, and so Henry Cavendish gets the credit. In 1766 he collected the bubbles and showed that they were different from other gases. He later showed that when hydrogen burns it forms water, thereby ending the belief that water was an element. The gas was given its name hydro-gen, meaning water-former, by Antoine Lavoisier.
Where is hydrogen in the periodic table?
Hydrogen element is in group 1 and period 1 of the Periodic table. Hydrogen is placed along with the alkali metals group as it has the similar outer electron configuration as that of the group 1 elements (i.e ns1).
Why is hydrogen in the 1st group?
Apart from having similar properties as that of group 1 and group 17 elements, hydrogen element is placed in 1st group because it has similar outermost electron configuration as that of alkali metals (i.e ns1).
Why is hydrogen gas used in balloons?
Hence due to its light-weight, it always moves upward in the atmosphere. Because of the light weight of hydrogen gas, it is also used in party balloons.
What is the mass of an atom?
Explanation: The mass of an atom completely depends on the number of protons and number of neutrons present in the nucleus of an atom. Protons and neutrons are the heavy particles in an atom. The mass of protons and neutrons is the same and it is 1.6749 × 10 -27 kg.
How much hydrogen is in the air?
There is around 0.000055 % of hydrogen in the air.
How many electrons are in a hydrogen atom?
Now in the Hydrogen atom, there is only one proton and only one electron.
Is hydrogen a flammable gas?
Yes, hydrogen is flammable and explosive too. For example, if you will give fire to the pure hydrogen balloon, then it will easily catch the fire and it will burn with yellow-orange flames. Hence hydrogen is flammable gas. But hydrogen is explosive when it is mixed with oxygen gas.
Why is hydrogen the first element on the periodic table?
Hydrogen is defined as the first element of the periodic table because its atomic number is 1, which means it contains only one single electron in its atom. Therefore only 1 electron is available in its outermost shell. The elements' placement in the periodic table is according to their electronic configuration.
Where is hydrogen on the periodic table?
Generally, in the periodic table, Hydrogen does not have a fixed position. In a few tables, it is placed with alkali metals (which is above Sodium), and in few others, it is lonely placed at the top (Randomly, Just above the first Period).
How many valence electrons does hydrogen have?
Hydrogen holds one valence electron in its outermost shell, and therefore it contains similar chemical properties compared to alkali metals. Also, hydrogen exists as a diatomic molecule similar to halogens and produces compounds with both metals and nonmetals. Thus, the hydrogen molecule can be placed in both the 1st as well as ...
What happens when a hydrogen atom loses an electron and produces a cation?
When a hydrogen atom loses an electron and produces a cation, it resembles the alkali metals whereas, when it gains an electron and becomes a uni-negative ion, it represents similarity to the halogens. By taking a look at these properties, the position of hydrogen in the periodic table is the major question.
What is the structure of hydrogen?
Structure of Hydrogen. The structure of hydrogen is similar to that of alkali metals (ns1), which contains one electron in their outermost shell. Also, it can attain helium noble gas configuration by accepting an electron. This character is mostly the same as that of the halogen family (ns2, np5), and is also short of one electron for ...
What is hydrogen used for?
Answer: Hydrogen is used in the glass industry to make flat glass sheets as a protective atmosphere. It can also be used as a flushing gas in the electronics sector while manufacturing silicon chips. And, the density with low hydrogen made it a natural choice for one of its first practical applications, which is filling airships and balloons.
What happens to the nucleus of a hydrogen atom when it loses electrons?
When the hydrogen atom loses electrons, the size of its nucleus decreases and almost becomes 1.5 × 10-3 pm, which is much smaller when compared to the atomic sizes of the normal metals, and therefore the hydrogen ion does not freely exist in nature.
Why is the position of hydrogen uncertain on the periodic table?
Why is the position of hydrogen uncertain on the periodic table?#N#Ans: The position of hydrogen is uncertain in the periodic table because some of the properties of hydrogen resemble that of the elements of the alkali metals, and some of the properties of hydrogen resemble that of halogens.
What type of compounds does hydrogen combine with?
Like halogens, hydrogen combines with non-metals like C, S i, G e, etc., to form the similar type of covalent compounds.
How do hydrogen and alkali metals form monopositive ions?
Both hydrogen and alkali metals can easilyform mono-positive ions by losing their single valence electron.
What is the name of the compound that combines with alkali and alkaline earth metals to form?
Hydrogen combines with alkali and alkaline earth metals to form hydrides which are similar to the corresponding compounds of halogens known as halides. For example, N a H, and N a C l, C a H 2 and C a C l 2 etc.
How many electrons does a halogen atom need?
Halogen atoms possess seven electrons in their valence shells and need only one electron to attain a stable configuration similar to those of noble gases. Similarly, the hydrogen atom also needs one electron to attain a stable configuration.
Which element is in group 1?
Hydrogen is an element that possesses unique properties. It has one electron in its valence shell similar to alkali metals and resembles them in several properties. Therefore, it should be placed in group 1 along with the alkali metals. On the other hand, like halogens of group 17, only one electron needs to attain the stable noble gas configuration. Hence, it also resembles halogens and can be grouped with them in group 17. Thus, the position of hydrogen is debatable.
Is hydrogen a gas?
Hydrogen is the lightest element of the periodic table and is colourless, odourless, and highly flammable gas. It is the most abundant element found in the universe. Hydrogen occurs as a diatomic molecule in nature as H 2. The molecule of hydrogen has a single covalent bond within both the hydrogen atoms. The electronic configuration is 1, which means that there is one electron present in the K -shell. It has 1 valence shell having 1 electron as well, as it shows a valency of 1. Also, the electronic configuration according to shell distribution – 1 s 1.
When was hydrogen discovered?
Hydrogen is one of the three most abundant elements present on Earth. It was discovered in 1766 by Henry Cavendish and is widely used for various industrial, medical and recreational purposes.
Where is hydrogen found?
Hydrogen is found in significant abundance in undetected form of mass, such as dark matter, and in gas giant planets and stars. In gaseous form, it is present only in a small fraction and makes around 1 part per million in volume.
How many isotopes of hydrogen are there?
There are three naturally occurring isotopes of hydrogen, named 1 H (protium), 2 H (deuterium), and 3 H (tritium). Protium is the most abundant hydrogen isotope (99.98%). Tritium is the most stable radioisotope and have a half-life of 12.32 years. All heavier isotopes of hydrogen are synthetic and are extremely unstable.
What is the most common elemental compound produced by hydrogen peroxide?
One of the most common compounds produced by elemental hydrogen is hydrogen peroxide (H 2 O 2) with oxygen. The light atomic nature of hydrogen impart various favorable properties to it, including low viscosity, and high thermal conductivity and specific heat as compared to all other gases.
What is the reaction of free hydrogen radicals?
Free hydrogen radical can react with chlorides and oxides of various metals to form free metals. It can lead to the formation of hydrides of various metals, non-metals such as, H 2 S, NAH, PH 3 and KH. One of the most common compounds produced by elemental hydrogen is hydrogen peroxide (H 2 O 2) with oxygen.
What is hydrogen used for?
Hydrogen is used as rocket fuel and as rocket propellent. Hydrogen provides protective and stable atmosphere for the production of flat glass sheets in glass industry. It is used in electronic industry for the manufacturing of silicon chips (as flushing gas). Hydrogen is widely used in power stations as a coolant in generators.
What happened to the Hindenburg airship?
Hydrogen also has a notorious event related to its name, the incident of Hindenburg airship (1937), were combustion of hydrogen led to the destruction of the ship in the midair and brought an end to the era of hydrogen-based air-travels [2].
How is hydrogen produced?
Hydrogen element is processed in several ways. They can be acquired to be consumed as hydrogen fuel cells. It can also be produced through industrial processes. Other methodological include biology hydrogen processing that is called electrolysis. Hydrogen steam reforming are also applied by many industrials plants. The most common technique for acquiring this element is steam reformation. Steam reacts when mixed with methanol to produces carbon monoxide and hydrogen.
What is the reaction of hydrogen protons?
Reactions of hydrogen protons is what produces light or other energy which allows the universe remains active to this day. Even the sun’s energy itself is a direct result from the fusion of hydrogen to form helium. And the energy in the sun is enormous. However, hydrogen does not play a peculiarly essential role.
Why is hydrogen used in weather balloons?
However, it reacts highly with oxygen to form water. Because hydrogen element is lightweight, then scientists are able to fit it within weather balloons. Meteorologists weather balloons have this element filled to the brim in the balloons. These balloons are tied with instrumentation to record important information to analyze the climate.
What is the lightest gas?
The lightest of all known gases, hydrogen mixed with other chemical compounds and sometimes exponentially to form chemical compounds. Hydro gen element is applied for creating several important chemical compounds. Aside from ammoniac, hydrogen can be utilized in some other ways.
Why is hydrogen used in cars?
In recent years, hydrogen is applied to detect gas leakage that occurs. As gas leaks that may occur in power plants or in automotive vehicles, which although small gas leaks would still result in serious injury, especially if gas leak react with other chemical elements.
Why is hydrogen not reactive?
This is because in the normal conditions hydrogen is not reactive so it does not produce odors or certain colors that can be recognized by the human senses. Although hydrogen gas is the most abundant element, atomic mass of hydrogen is the lowest among other gases.
What is the most abundant element in the universe?
Hydrogen is the lightweight and most widely distributed element in the universe, it is colorless, odorless and tasteless gas. It has the lowest density of all gases combined. It has atomic number of 1 and in elemental processing hydrogen is often generated. Hydrogen is an important element for human life. It is spread in water and in almost all the molecules in organics material. It is the most abundance gas in the universe. Hydrogen or symbolized by H2 has a number of very abundant chemical properties.