
Who invented the periodic table?
Who published the first periodic table?
How did Mendeleev and De Chancourtois organize elements?
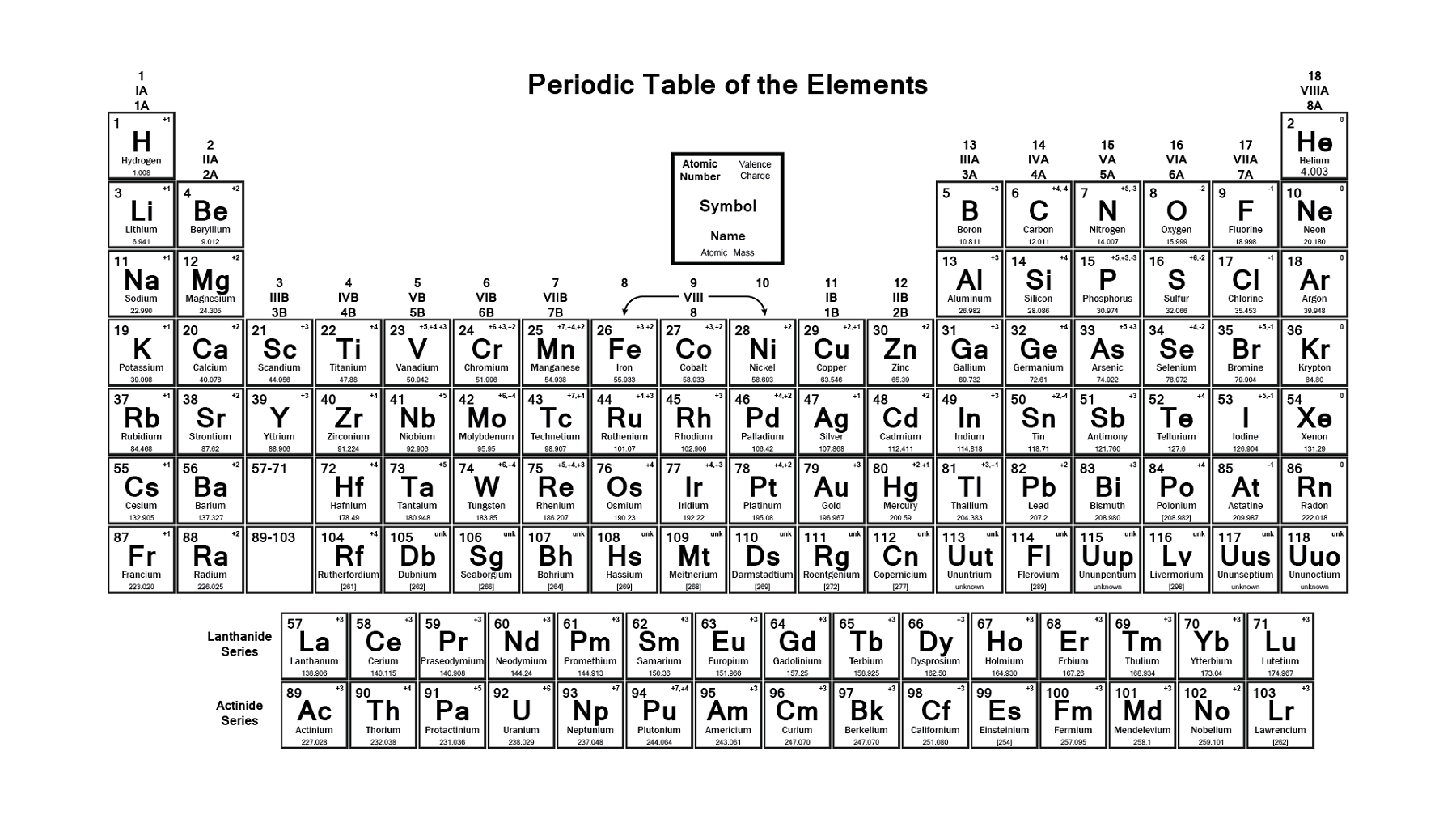
How is the periodic table arranged?
How does the periodic table order the elements?
When did Chancourtois publish his arrangement of elements?
What elements were discovered in Mendeleev's table?
See 4 more
About this website
Who developed the first truly periodic table?
MendeleevMendeleev discovered the periodic table (or Periodic System, as he called it) while attempting to organise the elements in February of 1869.
Who came up with the first periodic table and how?
In 1863 English chemist John Newlands divided the then discovered 56 elements into 11 groups, based on characteristics. In 1869 Russian chemist Dimitri Mendeleev started the development of the periodic table, arranging chemical elements by atomic mass.
What did John Newlands discover about the periodic table?
He arranged the known elements in order of increasing atomic weight, and found that elements with similar properties occurred at regular intervals. He divided the elements into seven groups of eight, in what he later called the 'law of octaves'.
When was the first periodic table made?
1869Science classrooms around the world hang periodic table posters on their walls. You can buy coffee mugs, pillowcases, even swimsuits adorned with the table. None of those representations look very much like the first periodic table, published in 1869.
How did Mendeleev discover the periodic table?
After his dream, Mendeleev drew the table he had envisioned. While arranging these cards of atomic data, Mendeleev discovered what is called the Periodic Law. When Mendeleev arranged the elements in order of increasing atomic mass, the properties where repeated.
How did Henry Moseley arrange the periodic table of elements?
Moseley organized his table in order of increasing atomic number . While atomic mass and atomic number generally correlate, because some elements have more, neutron heavy isotopes than others, they can have a higher atomic mass despite having a lower atomic number.
How was the first periodic table arranged?
Mendeleev arranged the elements in order of increasing weight and broke them into rows such that elements in each column shared valence, the number of other atoms they combined with, as well as other properties.
How did the Russian scientist first arranged the elements?
Dmitri Mendeleev devised the periodic classification of the chemical elements, in which the elements were arranged in order of increasing atomic weight.
7 Chemists Who Contributed in the Development of the Periodic Table
Dmitri Mendeleev; The first chemist who designed periodic table is Dmitri Mendeleev. He is a Russian chemist who first forumlated Periodic Law. When he first saw some of the discovered chemicals, he decided to create a better table to classify those elements into a more coherent table.
History of the periodic table - Wikipedia
The history of the periodic table is also a history of the discovery of the chemical elements.The first person in recorded history to discover a new element was Hennig Brand, a bankrupt German merchant. Brand tried to discover the philosopher's stone—a mythical object that was supposed to turn inexpensive base metals into gold. In 1669, or later, his experiments with distilled human urine ...
Who discovered the periodic system?
Important forward steps were the formulation of the general rules of the old quantum theory by William Wilson and Arnold Sommerfeld in 1916, the discovery of the exclusion principle by Wolfgang Pauli in 1925, the discovery of the spin of the electron by George E. Uhlenbeck and Samuel Goudsmit in 1925, and the development of quantum mechanics by Werner Heisenberg and Erwin Schrödinger during the same year. The development of the electronic theory of valence and molecular structure, beginning with the postulate of the shared electron pair by Gilbert N. Lewis in 1916, also played a very important part in explaining the periodic law ( see chemical bonding ).
Who suggested that the atomic number be identified with the ordinal number of the element in the periodic system?
In 1911 A. van den Broek suggested that this quantity, the atomic number, might be identified with the ordinal number of the element in the periodic system (following the lead of Newlands, it had become customary to number the elements according to their position in the table).
How many elements are in the periodic table?
Based on an earlier (1882) model of T. Bayley, J. Thomsen in 1895 devised a new table. This was interpreted in terms of the electronic structure of atoms by Niels Bohr in 1922. In this table there are periods of increasing length between the noble gases; the table thus contains a period of 2 elements, two of 8 elements, two of 18 elements, one of 32 elements, and an incomplete period. The elements in each period may be connected by tie lines with one or more elements in the following period. The principal disadvantage of this table is the large space required by the period of 32 elements and the difficulty of tracing a sequence of closely similar elements. A useful compromise is to compress the period of 32 elements into 18 spaces by listing the 14 lanthanoids (also called lanthanides) and the 14 actinoids (also called actinides) in a special double row below the other periods.
What is the long period form of the periodic system?
Long-period form of periodic system of elements. Encyclopædia Britannica, Inc. With the discovery of the noble gases helium, neon, argon, krypton, radon, and xenon by Lord Rayleigh (John William Strutt) and Sir William Ramsay in 1894 and the following years, Mendeleyev and others proposed that a new “zero” group to accommodate them be added to ...
What elements did Mendeleyev predict?
Mendeleyev was also able to predict the existence, and many of the properties, of the then undiscovered elements eka-boron, eka-aluminum, and eka-silicon, now identified with the elements scandium, gallium, and germanium, respectively. Similarly, after the discovery of helium and argon, the periodic law permitted the prediction of the existence ...
How does the atomic weight of an element show its position in the periodic system?
That the exact atomic weight of an element is of small significance for its position in the periodic system is shown by the existence of isotopes of every element —atoms with the same atomic number but different atomic weights. The chemical properties of the isotopes of an element are essentially the same, and all the isotopes of an element occupy the same place in the periodic system in spite of their differences in atomic weight.
Which elements were put in positions out of the order of atomic weights?
In the pairs argon and potassium, cobalt and nickel, and tellurium and iodine, for example, the first element had the greater atomic weight but the earlier position in the periodic system. The solution to this difficulty was found only when the structure of the atom was better understood.
Who found the periodic table?
It has been the go-to reference on chemical elements for almost 150 years. Yet while the Russian chemist Dmitri Mendeleev is often credited with finding the rules behind the block-like patterns of elements, he was hardly alone: others had found them some years before, but failed to win recognition.
What did Mendeleev discover?
His confidence was vindicated with the discovery of gallium, germanium and scandium, ensuring his place among the great names of 19th-Century science.
Who discovered that elements with similar properties lie close together?
One of these scientists was John Newlands , an English chemist who in the mid-1860s pointed out that elements with similar properties lie close together if arranged according to their atomic mass.
Who invented the periodic table?
But it was the combined efforts of many chemists for the invention of Periodic table. The chemists who invented Periodic table are listed below. Antoine Lavoisier (1789) Johann Dobereiner (1829) Alexandre Beguyer de Chancourtois (1862) ...
Who was the first person to publish the periodic table?
Dmitri Mendeleev (1869) Henry Moseley (1913) And many more…. Many of you might think that Mendeleev was the person who discovered the Periodic table. But this is not completely true. Yes, he was the first to publish the Periodic table with 63 elements which were discovered during his time.
Why Periodic table was invented?
The Periodic table was invented in order to classify all the known elements according to the similarities in their properties.
How did Johann Dobereiner classify the elements?
He classified the known elements by knowing their properties. After few years of this classification, several attempts were also made by other chemists. But some important work was given by Johann Dobereiner after few years. Let us see how Johann Dobereiner contributed to the development of Periodic table.
What did the king prepare for each known element?
He prepared the cards of each known element with their properties and details written on them.
How many elements are there in the universe?
Till today there are total 118 known elements like hydrogen, helium, lithium, beryllium, boron, carbon, and so on…
When did the periodic table start?
I’ll tell you the complete History of Periodic table starting from 1789 to 1913.
Who created the periodic table?
The periodic table was invented by Russian chemist Dmitri Mendeleev in 1869. However, prior to Mendeleev, chemists had been pondering for decades how to classify the elements. Beginning in 1789, Antoine Lavoisier began classifying elements by their properties. Johann Wolfgang Döbereiner showed in 1817 that elements could be arranged by their atomic ...
How is an element's position on the periodic table determined?
It was not until the early 20th century that it was discovered that an element’s position in the periodic table is determined by its atomic number (the amount of protons in its atomic nucleus).
Who used atomic weights to classify the elements?
Atomic weights were used by English chemist John Newlands in 1864 in classifying the elements. After arranging the elements in order by atomic weight, Newlands noted that every eighth element seemed to have similar chemical properties. By analogy with the seven-note musical scale, he called this the law of octaves.
How many columns did Mendeleev have in his 1869 table?
His 1869 table contained 17 columns (or groups, as they are now known). He revised this into an eight-group table in 1871. In his 1871 table, Mendeleev correctly predicted that the then known atomic weights of 17 elements were wrong.
Who was the first to arrange the elements into a periodic table with increasing order of atomic masses?
Dmitri Mendeleev. Lothar Meyer. British chemist John Newlands was the first to arrange the elements into a periodic table with increasing order of atomic masses. He found that every eight elements had similar properties and called this the law of octaves.
Who created the table of elements?
Among the scientists who worked to created a table of the elements were, from left, Antoine Lavoisier, Johann Wolfang Döbereiner, John Newlands and Henry Moseley. In 1789, French chemist Antoine Lavoisier tried grouping the elements as metals and nonmetals.
What elements did Mendeleev predict?
The later discovery of elements predicted by Mendeleev, including gallium (1875), scandium (1879) and germanium (1886), verified his predictions and his periodic table won universal recognition. In 1955 the 101st element was named mendelevium in his honor. The 1869 periodic table by Mendeleev in Russian, with a title that translates "An experiment ...
Why is the periodic table important?
The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities between them. Scientists use the table to study chemicals and design experiments. It is used to develop chemicals used in the pharmaceutical and cosmetics industries and batteries used in technological devices.
What are the horizontal rows in the periodic table called?
In the periodic table, the horizontal rows are called periods, with metals in the extreme left and nonmetals on the right. The vertical columns, called groups, consist of elements with similar chemical properties. The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities ...
What is the periodic table of chemical elements?
On its website marking the celebration, UNESCO wrote, “The Periodic Table of Chemical Elements is more than just a guide or catalogue of the entire known atoms in the universe; it is essentially a window on the universe, helping to expand our understanding of the world around us.”.
Why is the periodic table celebrated in 2019?
UNESCO named 2019 the International Year of the Periodic Table to mark the 150 th anniversary of Mendeleev’s publication. Researchers and teachers worldwide took this opportunity to reflect on the importance of the periodic table and spread awareness about it in classrooms and beyond.
When was the first periodic table created?
The first created periodic table was in 1863 where there were 56 discovered elements. At the very first he was just trying to write a text book for his students when he realized that there is pattern between the elements. After that he attempted to classify the elements and created his first periodic table.
Who created the periodic table?
Here is the list of them. Dmitri Mendeleev. The first chemist who designed periodic table is Dmitri Mendeleev. He is a Russian chemist who first forumlated Periodic Law. When he first saw some of the discovered chemicals, he decided to create a better table to classify those elements into a more coherent table.
Why is the periodic table important?
It is because each element discovered by different chemist. The periodic table has the chemicals or elements in ordered atomic number.
What chemicals went against atomic number?
These chemicals are iodine, tellurium and many more. Beside that, he conducted experiment by firing x ray gun to some samples of the elements. Because of this experience he found out that some chemicals go against completely atomic number and they created perfect straight line by that. Because of this, Henry Moseley completed the developing periodic table.
Why is it easier to classify metals?
After that he drew the pattern periodically and drew the line that connects the dot of the particular elements with similar atomic weight. By now it gets easier to classify elements such as metal or non metal because of his periodic arrangement.
Why were gases rare?
Gases were rare case of discovery because of its unapparent nature. But with his way of isolating argon and other chemicals, he successfully created new section in the current periodic table. This marks him as one of the most influential chemist. His discovery also leads him prize of Nobel in chemistry that further prove him to be excellent chemist.
What element is named after a human?
For his contribution in chemistry, the element of 106 later gets renamed as seaborgium. This element is the only one named after human. Related to Chemists Who Worked on The Manhattan Project.
Who invented the periodic table?
Most people think Mendeleev invented the modern periodic table. Dmitri Mendeleev presented his periodic table of the elements based on increasing atomic weight on March 6, 1869, in a presentation to the Russian Chemical Society.
Who published the first periodic table?
A year earlier (1864) Lothar Meyer published a periodic table that described the placement of 28 elements. Meyer's periodic table ordered the elements into groups arranged in order of their atomic weights. His periodic table arranged the elements into six families according to their valence, which was the first attempt to classify the elements according to this property.
How did Mendeleev and De Chancourtois organize elements?
Both de Chancourtois and Mendeleev organized elements by increasing atomic weight. This makes sense because the structure of the atom was not understood at the time, so the concepts of protons and isotopes had yet to be described.
How is the periodic table arranged?
While Mendeleev and Chancourtois arranged elements by atomic weight, the modern periodic table is ordered according to increasing atomic number (a concept unknown in the 19th century.)
How does the periodic table order the elements?
The modern periodic table orders the elements according to increasing atomic number rather than increasing atomic weight. For the most part, this doesn't change the order of the elements, but it's an important distinction between older and modern tables.
When did Chancourtois publish his arrangement of elements?
In 1862 (five years before Mendeleev), de Chancourtois presented a paper describing his arrangement of the elements to the French Academy of Sciences. The paper was published in the Academy's journal, Comptes Rendus, but without the actual table.
What elements were discovered in Mendeleev's table?
Some elements were known since ancient times, such as gold, sulfur, and carbon. Alchemists began to discover and identify new elements in the 17th century.

Why Periodic Table Was invented?
Who Invented Periodic table?
- It was not the efforts of a single person (i.e Dimitri Mendeleev) behind the invention of Periodic table. It was the combined efforts of many scientists who contributed to the discovery of the Periodic table. Following chemists gave their valuable efforts in creating the Periodic table. 1. Antoine Lavoisier (1789) 2. Johann Dobereiner (1829) 3. Ale...
Final Arrangement
- After the valuable contribution of all the above mentioned scholars, many researchers worked on the atomic structure and finally the structure of atoms came into existence. Scientists realized that there is a heavy nucleus in the centre of atoms. This nucleus contains protons and neutrons in it. They also realized that the protons are the unique identity of every single element. The num…
Free Gift For You: Interactive Periodic Table
- Let me tell you how this Interactive Periodic Tablewill help you in your studies. 1).You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).You will get the detailed information about the periodic table which will convert a newbie into pro. 3).You will also get the HD images of the Periodic table (for FREE). Checkout Interactive Per…