What is 17 on the periodic table?
What is 17 on the periodic table? Chlorine is in group 17 of periodic table, also called the halogens, and is not found as the element in nature – only as a compound. The most common of these being salt, or sodium chloride, and the potassium compounds sylvite (or potassium chloride) and carnallite (potassium magnesium chloride hexahydrate).
What is the Order of elements in the periodic table?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
What are the numbers on the periodic table?
What are the numbers in the periodic table? The number above the symbol is the atomic mass (or atomic weight). This is the total number of protons and neutrons in an atom. The number below the symbol is the atomic number and this reflects the number of protons in the nucleus of each element’s atom. There are 18 main columns of elements in the ...
What is N on the periodic table?
The seventh element in the periodic table is nitrogen. The nitrogen atom contains a total of seven electrons and protons. Therefore, the atomic number of nitrogen (N) is 7. Nitrogen is a p-block element and its symbol is ‘N’. This article discusses the properties of the nitrogen element and the importance of the nitrogen atomic number.

What are the 7 rows on the periodic table?
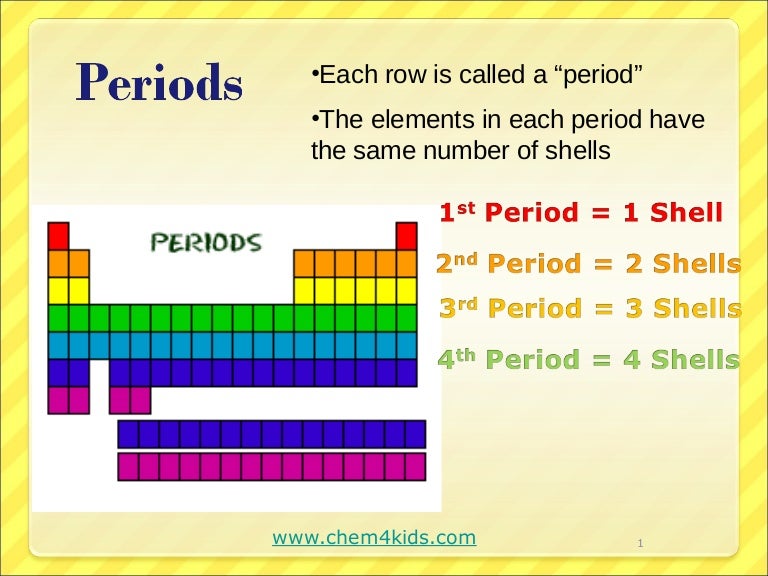
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups.
What are rows of elements called?
As with any grid, the periodic table has rows running left to right, and columns running up and down. The rows are called PERIODS and the columns are called GROUPS.
What is a row in a periodic table?
When you look at the periodic table, each row is called a period (Get it? Like PERIODic table.). All of the elements in a period have the same number of atomic orbitals. For example, every element in the top row (the first period) has one orbital for its electrons.
What is the first row of the periodic table?
The first row has two elements—hydrogen and helium.
What do rows and columns mean in periodic table?
The columns of the periodic table are called groups. Members of the same group in the table have the same number of electrons in the outermost shells of their atoms and form bonds of the same type. The horizontal rows are called periods.
What are rows and columns?
A row can be defined as an order in which objects are placed alongside or horizontally. A column can be defined as a vertical division of objects on the basis of category. The arrangement runs from left to right. The arrangement runs from top to bottom.
What are the horizontal rows called?
periodsThe horizontal rows are known as periods and vertical columns in the periodic table are known as groups.
Are rows in the periodic table called periods?
Periods are the horizontal rows of the periodic table. There are seven periods total and each element in a period has the same number of atomic orbitals. The top period, which contains hydrogen and helium, has only two orbitals. As you go down the rows, the number of orbitals increases.