The period number on the Periodic table tells you the total number of orbits that the atom An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small; typical sizes are around 100 picometers (1×10⁻¹⁰ m, a ten-milliont…Atom
What do the whole numbers on the periodic table represent?
What does the whole number on the periodic table represent? Atomic Number First, there is an integer (whole number) in some part of the box. This is the atomic number of the element. It represents the number of protons found in the nucleus of one atom of that particular element.
What number is periodic table arranged by?
The Periodic table elements are arranged in the increasing order of their atomic number. The arrangement of elements in the Periodic table starts from the very first top left corner. The first element with atomic number 1 (i.e hydrogen) is placed in the first cell, then gradually the elements with atomic number 2, 3, 4 upto 118, are placed from the left to right in the Periodic table .
What does the period number on the periodic table represent?
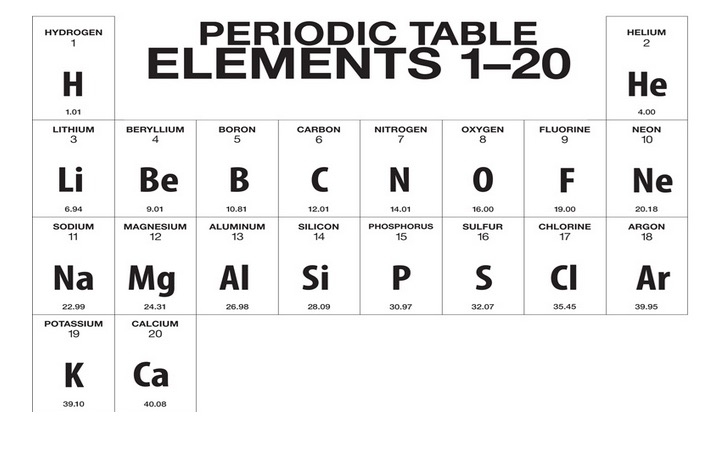
- The number above the symbols is atomic number of element.
- The number below the symbols is atomic mass of the element.
- The number above the boxes from left to right is the group (vertical columns) number.
- The number on the left side of periodic table is the period (horizontal rows) number.
What does periodic table tell us?
The Periodic Table offers basic information about each one of the known chemical elements. Each element has its own box in the table, and these boxes include the element's atomic number, atomic weight and chemical symbol. An element's position on the table indicates which elements share its basic properties.

How do you read the numbers on the periodic table?
When you're reading the periodic table, move across the table from top left to bottom right. As you move across the table, the number of protons and the atomic mass of each element increases. Each element has its own atomic number, which represents the number of protons in one atom of the element.
What do the three numbers on the periodic table mean?
Elements and Symbols These three pieces of data are the elemental symbol, the atomic number (typically given the symbol, Z, and the atomic weight.
How do you read groups and periods on the periodic table?
The columns of the periodic table are called groups. Members of the same group in the table have the same number of electrons in the outermost shells of their atoms and form bonds of the same type. The horizontal rows are called periods.
How do you read a periodic table box?
0:103:58Read the Periodic Table of Elements - YouTubeYouTubeStart of suggested clipEnd of suggested clipBased on its atomic structure the elements are organized by atomic number which represents theMoreBased on its atomic structure the elements are organized by atomic number which represents the number of protons of each element. From the element with the lowest atomic.
What do the numbers on the periodic chart mean quizlet?
The number (usually placed) above the element symbol, that tells you how many protons, and how many electrons are in the atom.
What do the numbers on the left side of the periodic table mean?
On the periodic table, the number in the left corner is the number of protons, the number in the right corner is the atomic weight, the Symbol for the element is in the center, and the name is on the bottom. Atomic. number. The number of protons, unique to each element, in.
What is the name of groups 3 12?
the transition metalsThe elements in groups 3-12 are called the transition metals.
What group number means?
Group number signifies the number of electrons in the outermost shell of an atom of the element. The number of electrons in the outer shell of all elements in one group is same, which implies that. They have the same valency and same chemical properties. Related Questions.
What is atomic number?
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide...
What is the atomic number and mass number?
The number of protons and the number of neutrons shall determine the mass number of an element. Since the isotopes of an element have slightly diff...
Can two different elements have the same atomic number?
Atoms from two different elements may have the same neutron count, but never the same proton count. The number of protons is unique to the element...
How do we calculate atomic mass?
Add the mass of protons and neutrons to compute the atomic mass of a single atom of an element. Example: Find the atomic mass of a carbon isotope w...
Why is atomic number important?
Atomic number is called the number of protons in an atom. This number is very important, because it is unique to a given element’s atoms. An elemen...
What information is on the periodic table?
There are other periodic tables that have additional information on them such as the size of atoms, density of elements and melting points. Trends can be seen as you go along the rows and columns of the periodic table, which I may discuss in further detail in a future post. But normally the most basic ones have the atomic and mass numbers of each element on them, as well as the symbol used to abbreviate the element’s name.
Why is the periodic table important?
The periodic table is used daily by chemists and other scientists as reference resource for the ingredients of the universe. Ancient Greeks defined “elements” as one of the following four: earth, fire, water and air. They were used to explain how matter worked.
Why are the number of protons and electrons in a neutral atom the same?
The number of protons and electrons in a neutral atom are the same in order to balance the charge. An atom’s structure is made up of a central nucleus made up of protons and neutrons with electrons whizzing around the nucleus. Because the electrons are on the exterior of the atom they are the particles that are actually involved in chemical ...
What is the difference between atomic number and mass number?
The atomic number can be defined as the number of protons present in the central nucleus of the element’s atom while the mass number is the total number of protons and neutrons in the nucleus. The mass number is the larger of the two numbers found associated with an element in the table. The mass number can be quantified by a constant (a number ...
What color are elements in the periodic table?
Picture caption: periodic table coloured according to the phase (solid, liquid, gas) an element exists in at room temperature. Most elements are solid, coloured red; some gas (blue) and only two are liquid, bromine and mercury. Source: periodictable.com. Mendeleev chose to arrange the elements by atomic number rather than mass number ...
What are the elements that change as they get bigger?
As an element gets bigger – with more and more protons, neutrons and electrons packed into its structure – its properties change. The smaller elements at the top of the periodic table are gases like hydrogen (atomic number 1), helium (atomic number 2) and oxygen (atomic number 6) while the heavier elements towards the bottom of the table are metals like gold (atomic number 79), lead (atomic umber 82) and uranium (atomic number 92).
Why do electrons whizz around in the nucleus?
The presence of protons in the nucleus makes the nucleus overall positively charged and the electrons whizz around within the proximity of the nucleus because they , as negatively charged particles, are attracted to that positively charged nucleus.
How many elements are in the periodic table?
The table below consists of 118 elements of the periodic table, sorted by atomic number, atomic weight, symbols, density, discovered year and the group.
What is the atomic number of an element?
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of [He] 2s 2 2p 2, since its atomic number is 6.
What is the number of protons in the nucleus called?
The number of protons in the nucleus is called the atomic number. The atomic number of each element is unique.
Why is the atomic number of each element unique?
While the atomic number always stays the same some elements have atoms with different atomic mass numbers. This is because some elements have a different number of neutrons in the nucleus.
How to find the mass of an element?
The number of protons and the number of neutrons shall determine the mass number of an element. Since the isotopes of an element have slightly different mass numbers, it calculates the atomic mass by obtaining the mean of the mass numbers for its isotopes.
How can periodic trends be observed?
Periodic trends in the properties of the elements can be observed down the groups and across the periods of the modern periodic table. Every chemical element has a specific atomic number, which provides insight into the number of protons present within its nucleus.
Why is the atomic number important?
This number is very important, because it is unique to a given element’s atoms. An element’s atoms all have the same number of protons and each element has a different number of protons in its atoms. Test your knowledge on periodic table elements.