What are the properties of Group 17?
The atomic properties of group 17 which are going to be discussed are:
- Ionic and atomic radii
- Ionization enthalpy
- Electron gain enthalpy
- Electronegativity
What element is in Group 17 and period 2 of the periodic table?
fluorine (f) lies in group 17 and 2 period Other questions on the subject: Chemistry What was the procedure by which case united states vs lopez went to court... Chemistry
What are the groups and periods of the periodic table?
- Lithium (Li)
- Sodium (Na)
- Potassium (K)
- Rubidium (Rb)
- Cesium (Cs)
- Francium (Fr)
What are the 8 families of elements?
- alkali family. group 1, hydrogen is not a member.
- alkaline earth. group 2, also very reactive, only 2 valence electrons.
- transition metals.
- boron family.
- carbon family.
- nitrogen family.
- oxygen family.
- halogen family.
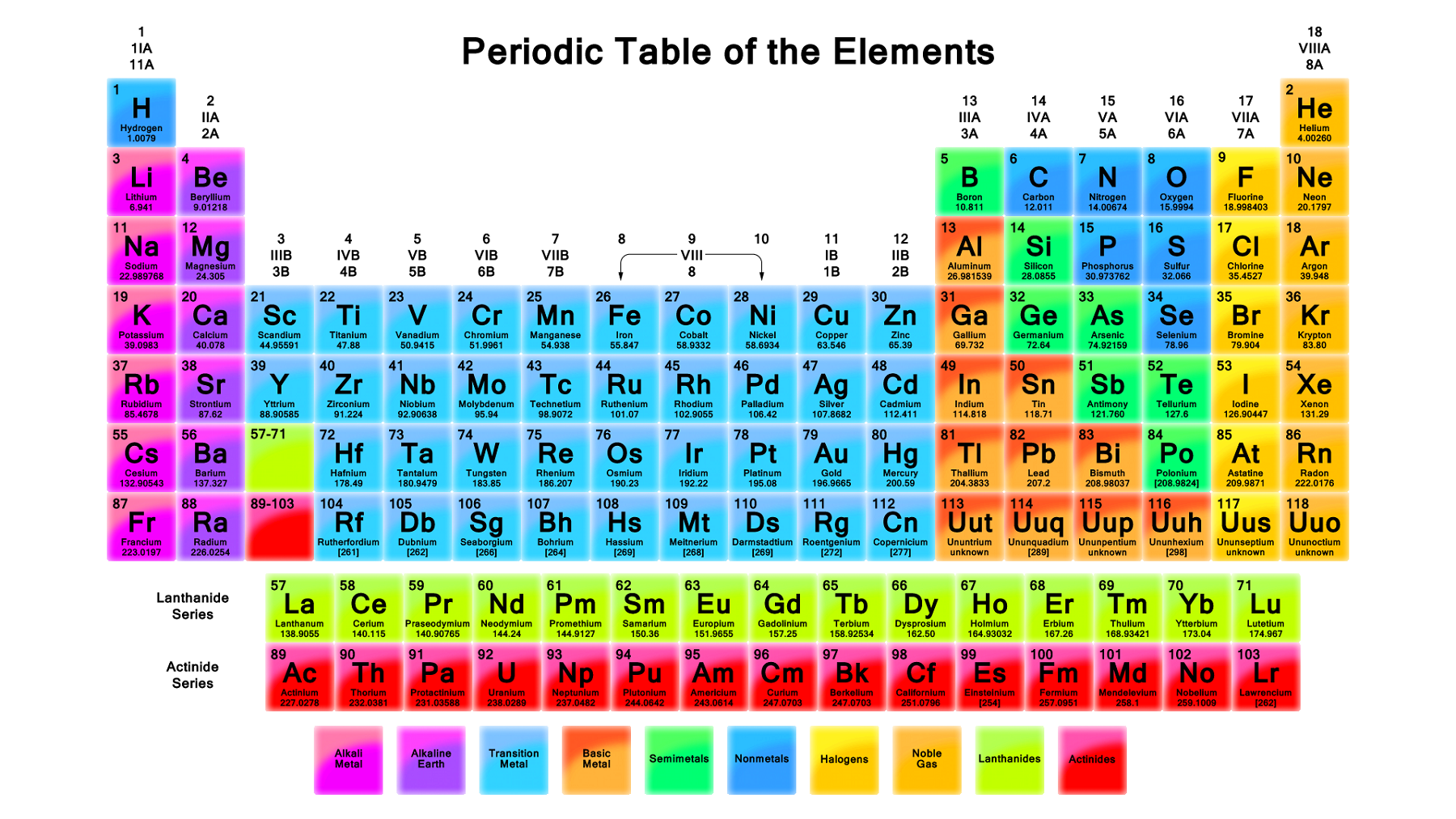
Why are Group 17 elements called that?
Group 17 elements are called halogens. The name halogens are from Greek halo (sea salt) and gens (producing formation) and thus means 'sea salt former'. Group 17 contains fluorine, chlorine, bromine, iodine which form salts. Hence they are called halogens.
What is Group 18 called in the periodic table?
noble gasnoble gas, any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).
What are Group 16 elements called?
chalcogenoxygen group element, also called chalcogen, any of the six chemical elements making up Group 16 (VIa) of the periodic classification—namely, oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po), and livermorium (Lv).
What are the group 13 to 17 elements called?
The main group elements of the periodic table are groups 1, 2 and 13 through 18. Elements in these groups are collectively known as main group or representative elements.
Why are Group 18 called noble gases?
These gases are unreactive or inert because they do not gain, lose, or share electrons. Because they are found in such small amounts in the earth's atmosphere, they are also known as noble gases.
What are the elements in Group 18 called and why?
Noble (Inert) Gases or Group 18 Elements. Noble or inert gases are elements in Group 18. They are named inert because they do not participate in any chemical reaction, hence they are chemically inert. Helium, Neon, Argon, Krypton, Xenon, and Radon are all non-metallic elements of group 18.
Are there 18 groups in the periodic table?
The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups.
Why are 18th group elements called inert gases?
The elements placed in group 18 have completely filled valence shells due to which they are non-reactive and called inert gases.
Why is I-Cl bond more polar reactive than I 2 ?
I-Cl bond is polar and weaker than I-I bond, so I-Cl is more reactive than I 2 .
Bleaching effect of Chlorine is permanent. Why?
It is due to the oxidation of coloured substances to colourless substances by nascent oxygen.
Compare the oxidising power of F 2 and Cl 2 .
The electrode potential of F 2 is maximum but that of I 2 is minimum. This means that F2 can be reduced very easily but I2 is reduced less readil...
Unlike chlorine, fluorine forms only one oxoacid, HOF.
Due to small size, high electronegativity and unavailability of d- orbital in fluorine.
Name two poisonous gases prepared from chlorine.
Mustard gas, phosgene, tear gas
What are the elements in Group 17?
Group 17 elements are collectively called as halogens (In Greek: halo means salt and genes mean producing, so collectively salt producing) and it consists of fluorine, chlorine, bromine, iodine, and astatine. The similarity to this extent is not found in other groups of the periodic table.
Why does ionization enthalpy decrease in group 17?
Thus, they have a very high value of ionisation enthalpy. Ionisation enthalpy decreases from top to bottom in the group due to the increase in atomic size.
Which element has a valence shell of +4 and +6?
The oxides and oxoacids of chlorine and bromine have +4 and +6 states. There are no valence shells d orbitals in fluorine atom and therefore it cannot expand its octet. Fluorine being the most electronegative element exhibits only -1 oxidation state.
Which element has the highest electronegativity?
The elements of group 17 have a very high value of electronegativity. The electronegativity decreases down the group due to the decrease in effective nuclear charge. Thus, fluorine is the most electronegative element.
How many electrons does Astatine have?
They have a regular gradation in the physical and chemical properties. Astatine is the only radioactive element in the group. They have seven electrons in their outermost shell (ns2np5) and are short of one electron from the configuration of the nearest noble gas.
Which family has a maximum negative electron gain enthalpy?
Electron Gain Enthalpy of Halogen Family. The atoms of group 17 elements are only one electron short of attaining stable noble gas configurations. Thus, these elements have a maximum negative electron gain enthalpy in the corresponding periods.
Which element has a oxidation state of -1?
All the elements of the halogen family exhibit -1 oxidation state. However, elements such as chlorine, bromine, and iodine also show +1, +3, +5 and +7 state.
Why are the elements in Group 17 categorised together?
Group 17 is the halogens, they have 7 electrons in their outermost shell. They are all non-metals , and form anions with a -1 charge. Reactivity decreases down the group, with fluorine being the most reactive.
What group form the P block?
Together with groups 13-16 and 18, they form the p-block.
Where was element 117 discovered?
Element 117 was discovered in a collaboration between the Joint Institute of Nuclear Research at Dubna, Lawrence Livermore National Laboratory in Livermore, California, Vanderbilt University, Tennessee, and Oak Ridge National Laboratory, Tennessee. A berkelium target was prepared in Oak Ridge and transported to Dubna where it was bombarded with calcium to produce six atoms of element 117 which were detected through their decay products. The results were announced in 2010. Further experiments between 2011 and 2014 confirmed the results of the earlier experiments and IUPAC recognised the discovery in 2015.
When was chlorine first discovered?
Chlorine compounds like salt have been used since ancient times, and chlorine gas was produced from the 1200s as a by-product of acid production, however its discovery as an element began in 1774 when Carl Wilhelm Scheele produced chlorine gas and called it dephlogisticated muriatic acid air. Scheele failed to recognise it as an element and it was eventually believed to be an oxide of the hypothetical element muriaticum. In 1809, Joseph Gay-Lussac and Louis-Jacques Thenard tried to release muriaticum from muriatic acid air and failed, raising the possibility that it was itself an element. The following year Sir Humphry Davy concluded that it is an element, which he named chlorine from the Greek chloros meaning pale green-yellow, which is the colour of chlorine gas. The alternative name halogen was proposed, meaning salt-producer, but instead came to refer to the group to which chlorine belongs.
What group are halogens in?
The halogens are a group of elements found in group 17 of the periodic table. Their name means salt-producer, and they are the most reactive non-metal elements.
How many valence electrons are in halogens?
Halogens have 7 valence electrons. This makes them very reactive as they seek to complete their outermost shell of 8 electrons.
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.