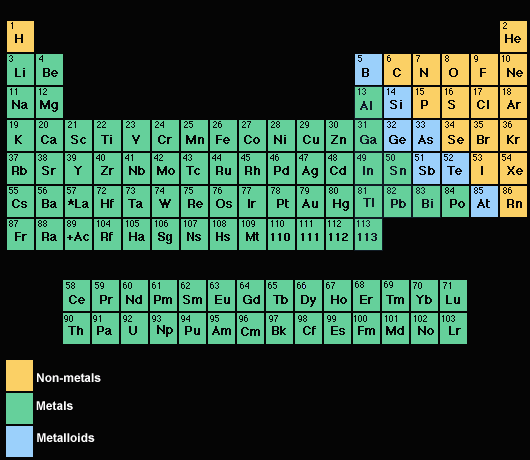
Which elements are the less reactive in the periodic table?
elements in group one of the periodic table which have one valence electron, most reactive metals, never found alone, shiny and soft Alkine earth metals Group 2 of the periodic table, melt at higher temps, a little less reactive than previous group transition metals
What metal is most active in the periodic table?
Lithium is the most active element in the periodic table. The periodic table is composed of 18 groups and 7 periods. In groups, it increases from left to right whereas, in periods, it increases from bottom to top. Where are the most active metals located 2 Where are the most active nonmetals located?
What is the most toxic element on the periodic table?
What is the most dangerous element in the periodic table? Plutonium Plutonium: A History of the World’s Most Dangerous Element. What element is the heaviest gas? Radon is the heaviest gas. It is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas.
What are the most active metals on the periodic table?
What are the top 10 most reactive elements?
- Aluminum. Atomic number is 13. It’s symbol is Al.
- Zinc. Iron’s symbol is Fe. It’s atomic number 26. Iron is on of the Earth’s most common element by mass. …
- Iron.
- Copper. Lead is located in the carbon group. Melting point is 625 degrees Fahrenheit. It’s symbol is Pb. …
What is the most reactive metal on the periodic table?
Which group of metals is the most reactive?
What Determines Reactivity?
Which element is more reactive, lithium or francium?
What happens to the atomic radius as you move down a column?
What is the purpose of the metal activity series?
See 3 more
About this website
Which periodic group of metals is most reactive?
alkali metalsThe most reactive metals in the periodic tables are the alkali metals, followed by the alkaline earth metals.
Which metals are most reactive in order?
In general, the alkali metals are the most reactive, followed by the alkaline earths and transition metals. The noble metals (silver, platinum, gold) are not very reactive....The reactivity series follows the order, from most reactive to least reactive:Cesium.Francium.Rubidium.Potassium.Sodium.Lithium.Barium.Radium.More items...•
What group is the most reactive metals group and why?
Alkali metals are among the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and have an oxidation state of +1. These metals are characterized by their soft texture and silvery color.
Where are the most reactive nonmetals on the periodic table?
Answer and Explanation: The most reactive nonmetals are the halogens (of group 17), which are located on the far right-hand side of the periodic table, just before the noble... See full answer below.
How do you determine the reactivity of metals?
We can use the reactions of metals with acids to tell us how reactive that metal is. The more vigorously it reacts, the more reactive the metal. The slower the reaction (sometimes there is no reaction at all), the less reactive the metal.
How do you determine the order of reactivity?
In a reactivity series, the most reactive element is placed at the top and the least reactive element at the bottom. More reactive metals have a greater tendency to lose electrons and form positive ions .
Why is group 17 the most reactive?
Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more reactive than other non-metal groups.
Why are groups 1 and 17 the most reactive?
These are the electrons in their outer energy level that can be involved in chemical reactions. Valence electrons determine many of the properties of an element, so elements in the same group have similar properties. All the elements in group 1 have just one valence electron. This makes them very reactive.
Is group 1 or group 2 more reactive?
Group 1 alkali metals are more reactive than group 2 Alkaline Earth Metals.
What is the most reactive metal and why?
Caesium, the most reactive metal in the periodic table, reacts extremely violently – hence why it can't be demonstrated in a classroom! This can be compared to other common metals, such as iron and copper, which produce no reaction when dropped into water.
Which of the following is the most reactive?
Xe is most reactive , as it has maximum ionization energy among given compounds. Also outer e− are loosely attracted by nucleus so it is reactive.Among the following inert gas elements, the elements that show highest chemical reactivity is: ... Thermal stability of hydrates of group 18 elements :More items...
How do you determine the most reactive nonmetal?
Fluorine is the most reactive non-metal because it is the most electronegative of all of the non-metal elements of the periodic table. Due to its strong electro negativity & small size, Fluorine has a strong tendency to accept electrons from other atoms or ions. As a result it oxidises all other substances.
What are the 10 least reactive metals?
The least reactive metals would be Platinum, Gold, Palladium, Osmium and Silver and in the decreasing order.
Which metal is more reactive iron or zinc?
Zinc more reactive than iron. The reason are: Zinc is a more electropositive element than iron. Zinc has a bigger atomic size than that of iron and thus has more number of electrons.
Which is more reactive iron or tin?
(iii) Zinc is more reactive than iron but tin is less reactive than iron.
What metal is the least reactive?
Silver, gold, and platinum are metals with the least reactivity.
Which is the most reactive metal: Caesium or Francium?
Caesium is more reactive. Generally as you go down the periodic table, electro-positivity increases. However, for the really heavy elements, the presence of so many positively charged protons in the nucleus has the affect of causing the electrons to move round at incredibly fast speeds approaching the speed of light.
What are the most Reactive Metals? | DocumentaryTube
The alkali metals are the family of elements that contain the most reactive elements. These metals include lithium, sodium, potassium, rubidium, cesium, and francium
Which group of metals is the most reactive?
They are the Alkali metals of group 1. In 1st group, as we move down from top to bottom, the reactive of metals increases. Thus the bottom most element of group 1 (i.e francium) is the most reactive metal on the Periodic table. ( Note: Francium is a laboratory made element.
Where are metals located on the periodic table?
The metals are located on the left side of the Periodic Table.
What are metals in Periodic table?
Metals are the elements which have the tendency to donate or lose electrons to form positive ions.
How many electrons do metals lose in a chemical reaction?
The atoms or metals have generally 1, 2 or 3 electrons in the outermost orbit, and they lose these electrons during a chemical reaction.
How many rare earth metals are there?
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Why do metals make a ringing sound?
Metals produce ringing sound when they are stuck hard. This indicates that metals are sonorous in nature.
What are the elements in group 3 to group 12?
The elements lying in group 3 to group 12 are known as Transition metals (or transition elements). Transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14. Thus these metals are known as “Transition metals”.
What is the most reactive metal on the periodic table?
Updated October 08, 2019. The most reactive metal on the periodic table is francium. Francium, however, is a laboratory-produced element and only minute quantities have been made, so for all practical purposes, the most reactive metal is cesium . Cesium reacts explosively with water, though it is predicted francium would react even more vigorously.
Which group of metals is the most reactive?
The most reactive metals belong to the alkali metals element group. Reactivity increases as you move down the alkali metals group. The increase in reactivity correlates to a decrease in electronegativity (increase in electropositivity.)
What Determines Reactivity?
Reactivity is a measure of how likely a chemical species is to participate in a chemical reaction to form chemical bonds. An element that is highly electronegative, such as fluorine, has an extremely high attraction for bonding electrons.
Which element is more reactive, lithium or francium?
So, just by looking at the periodic table, you can predict lithium will be less reactive than sodium, and francium will be more reactive than cesium and all of the other elements listed above it in the element group.
What happens to the atomic radius as you move down a column?
As you move down a column or group of the periodic table, the size of the atomic radius increases. For the metals, this means the outermost electrons becomes farther away from the positively-charged nucleus. These electrons are easier to remove, so the atoms readily form chemical bonds.
What is the purpose of the metal activity series?
You can use the metal activity series to predict which metal will be the most reactive and to compare the reactivity of different metals. The activity series is a chart that lists elements according to how readily the metals displace H 2 in reactions.
